Energy is available in various forms. We need different kinds of energy for doing different kinds of work. Quite often, a pariticular type of work can be done by using different forms of energy.
So, quite often we need to convert one form of energy into another. The change of one form of energy into antoher is known as transformation of energy.
Electrical energy is a very useful form of energy. It can be converted into chemical energy of certain types of substances. This is what we call as the chemical effects of current. In this chapter, you will learn about the processes in which electrical energy is converted into chemical energy.
* Defination: The phenomenon of causing chemical changes by passing electricity is called chemical effects of current.
* Electrodes: In a circuit an electrode is a conductor that is in contact with a nonmetallic thing like a liquid or gas.
* Cathode: The electrode connected to the negative terminal of a batlery is called the cathode.
![]()
* Anode: The electrode connected to the positive terminal of a battery is called the anode.
* Conduction in solids: All substances are made up of atoms, which have charged particles called electrons and protons. Electric current is produced due to movement of electron or charged particles.
Among solids metal are good conductor of electricity because electrons move randomly in different direction within the metal. When voltage is applied across a piece of a metal these electron move in one direction ot produce current.
* Conduction in Liquids: The current through them is constituted by the flow of electrons or ions.
Ions: An atom or a radical that becomes charged by losing or ganing one or more electrons is called an ion.
Cation: The positively charged ion formed by the loss of electon is called cation.
![]()
Anion: The negatively charged ion formed by the gain of electron is called anion.
![]()
anion
* Electrolyte: A liquid or a moist paste that has ions in it is called an electrolyte. Liquids that have ions. Such as acids and solutions of salts and bases conduct electricity. When a voltage is applied across electrodes placed in the electrolyte the ions start moving in orderly fasion. Cation move towars cathode and anion moves towards anode. Their flow constitute a current.
ELECTROLYSIS
The word electrolysis means breaking up by the action of electricity. It may be defined as follows:
The process of decomposition of an electrolyte with the help of electricity is called electrolysis.
For example, when electricity is passed through acidified water, using platinum electrodes, it decomposes into hydrogen and oxygen gases.
Electrolysis of water can be described by the equation,
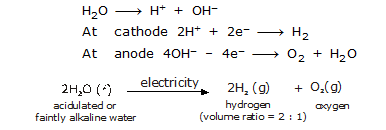
Application of Electrolysis:
The principle of electrolysis is employed in the following processes :
1. Manufacture of industrial chemicals 2. Extraction of metals
3. Refining of metals 4. Electroplating
1. Manufacture of Industrial Chemicals
Many chemicals which are used in industry in large quantities are prepared by electrolytic method.
Some of these are :
(i) Sodium hydroxide, NaOH (caustic soda) and Chlorine gas, Cl2(g) are prepared by the electrolysis of brine (20% common salt solution).
(ii) Hydrogen gas, H2(g) is prepared by the electrolysis of acidified water, or 20% NaoH solution. Oxygen is obtained as by-product.
2. Extraction of Metals–Electrometallurgy
More electropositive metals, such as sodium (Na), potassium (K), calcium (Ca), magnesium (Mg), aluminium (Al), etc. cannot be obtained by carbon reduction process. These metals can be obtained by the electrolysis of their molten chlorides, hydroxides or oxides.
For example,
1. Sodium and potassium are obtained by the electrolysis of their molten chlorides and hydroxides.
2. Calcium and magnesium are obtained by the electrolysis of their molten chlorides.
3. Aluminium is obtained by the electrolysis of its molten oxide (in the presence of some other compounds).
3. Refining of Metals
The metals obtained by chemical reduction methods generally contain many impurities. Such metals can be refined very easily by electrolytic method. The method of purifying metals by using electricity is called electrorefining. Metals, such as zinc, copper, silver, nickel, gold, aluminium, etc are refined by electrical method.
4. Electroplating
The process of depositing a thin layer of any superior metal over an object of a cheaper metal, with the help of electric current is called electroplating.
For example, deposition of silver on brass or copper objects and that of copper, nickel, chromium etc., on objects made of iron is done by electroplating.
Electroplating is done with the following purpose/objectives.
1. For decoration purposes : Silver or gold plating of brassware such as flower vase.
2. For preventing corrosion : Iron objects, such as, bathroom fittings etc., are electroplated with chromium.
* Process of Electroplating
The process of electroplating involves the followings steps :
1. Clean and wash the object to be electroplated thoroughly.
2. The object to be electroplated is made cathode.
3. A sheet of pure metal (to be electroplated) is made anode.
4. The electroplating tank is filled with the solution of a salt of the metal to be electroplated.
5. Connect the cathode to the negative (–) terminal and the anode to the positive (+) terminal of the battery.
6. Pass the current for a certain time to deposite a thin layer of the metal.
The process of electroplating is illustrated below.
* Electroplating of Copper
The experimental set up for electroplating copper on any object, such as a metallic spoon is shown in fig. given below :
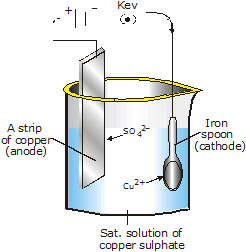
Here,
1. The object to be electroplated, say iron spoon is made cathode (–ve electrode).
2. A thin sheet of pure copper is made anode (+ve electrode).
3. An acidified solution of copper sulphate (CuSO4) is used as the electrolytic solution.
![]()
When electricity is passed through the solution, Cu+2 ions move towards the cathode and get reduced to copper metal. This copper metal gets electroplated on the objects, i.e., metallic spoon.
The sulphate ions (SO42–) move towards anode. Here SO42– ions do not get oxidised. Instead, the copper metal of the anode gets oxidised to Cu2+ions. These copper ions (Cu2+) go into the solution. As a result of this reaction at the anode, concentration of Cu2+ ions in the solution is maintained.
At cathode :
At anode :
ELECTROPLATING OF STEEL SPOON WITH SILVER
For electroplating a steel spoon with silver, a solution of a silver salt is taken as the electrolyte. The spoon and a silver bar are dipped into the electrolyte and connected to the negative and positive terminals of a battery. The positively charged silver ions move to the negative electrode(spoon) and form a deposit of silver on it.
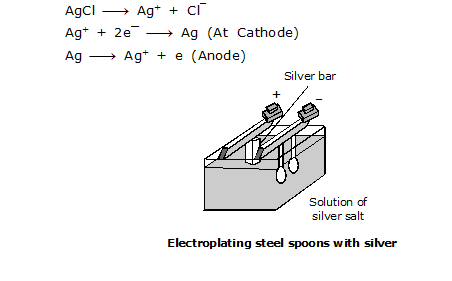
SOLVED EXAMPLES
Ex.1 What are conductors?
Sol. Materials which allow electric current to flow through them are called conductors.
Ex.2 What are insulators?
Sol. Materials which do not allow electric current to flow through them are called insulators.
Ex.3 Is distilled water a conductor of an insulator?
Sol. Distilled water is an insulator because there are no salts dissolved in it.
Ex.4 Classify the following liquids into conductors and insulators : lemon juice, distilled water, tap water, milk.
Sol. Conductors : lemon juice, tap water.
Insulators : distilled water, milk
Ex.5 Define electroplating?
Sol. electroplating is the process of depositing a layer of any desired metal on another metallic object by means of electricity.
Ex.6 Is air an insulator or conductor of electricity?
Sol. Air is an insulator of electricity.
Ex.7 What is LED?
Sol. LED is light emitting diode.
Ex.8 Name the gases formed when electric current is passed in water containing a few drops of acid?
Sol. Hydrogen and Oxygen
Ex.9 What is CFL?
Sol. Compact Fluorescent lamps.
Ex.10 What is an electric pen?
Sol. It is a device for writing on the surface with special compounds and material using the electrical property of ions.
Ex.11 When the free ends of a tester are dipped into a solution, the magnetic needle shows deflection. Can you explain the reason?
Sol. Yes, the solution does conduct electricity. Compass needle shows deflection due to magnetic effect of electric current.
Ex.12 Name three liquids, which when tested in the manner shown in figure, may causes the magnetic needle to deflect.
Sol. The compass needle will show deflection with tap water, lemon juice and sodium chloride solution.
Ex.13 A tester is used to check the conduction of electricity through two liquids, labelled A and B. It is found that the bulb of the tester glows brightly for liquids A while it glows very dimly for liquids B. You would conclude that
(i) Liquid A is better conductor than liquid B.
(ii) Liquid B is a better conductor than liquid A.
(iii) Both liquids are equally conducting.
(iv) Conducting properties of liquids cannot be compared in this manner.
Sol. (i) Liquid A is a better conductor of electricity because the bulb glows more in it.
Ex.14 Does pure water conduct electricity? If not, what can we do to make it conducting?
Sol. As pure water is free of salts and thus it is an insulator so to make pure water conducting, we can add some salts or acid to it.
Ex.15 In case of a fire, before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Sol. Firemen shut off the main electrical supply for the area because water is a good conductor of electricity and the firemen can get electrocuted.
Ex.16 Is it safe for the electrician to carry out electrical repairs outdoors during heavy downpour? Explain.
Sol. No, it is not advisable for wiremen to carry out electrical repairs during heavy downpour because water is a good conductor of electricity and the person can get shock.
Ex.17 A child staying in a coastal region tests the drinking water and also the seawater with his tester. He finds that the compass needle deflects more in the case of seawater. Can you explain the reason?
Sol. The sea water contains more salts dissolved in it as compared to the tap water. So, the deflection of the compass needle is more.
Ex.18 Paheli had heard that rainwater is as good as distilled water. So the collected some rainwater in a clean glass tumbler and tested it using a tester. To her surprise the found that the compass needle showed deflection. What could be the reasons?
Sol. The rainwater showed deflection with tester because it is not as pure as distilled water. Distilled water does not have any salts dissolved in it, but rain water may have some impurities in it.
Ex.19 Why the iron cans are electroplated with tin?
Sol. Tin is less reactive than iron. Thus food, stored in iron cans, is prevented from being spoilt by the iron by electroplating it by tin.
Ex.20 What is the disadvantage of electroplating done in the factories?
Sol. The disposal of the used conducting solution is hazardous and can cause environmental pollution. There are specific disposal guidelines for environmental protection.
Ex.21 The bulb does not glow in the setup shown in figure. List the possible reasons. Explain your answer.
Sol. It cannot be said for sure that liquid does not conduct electricity because :
(a) may be the cells are weak
(b) may be the current is so weak that it does not heat the filament of the bulb, so that it can glow.
Ex.22 How can you make a tester for testing an insulator?
Sol. A tester can be made by attaching one free connected to the material to be tested.
The bulb in the tester does not glow when current is passed through it. What can be the possible reasons for this?
Ex.23 The possible reasons for this are :
(a) the connectios may be loose
(b) the bulb may be defective.
(c) the cells may be defective
Sol. The liquid is a conductor and the circuit is also complete, but the bulb does not glow. Why?
Ex.24 The bulb does not glow even through the circuit is complete because the current is too small. The filament of the bulb is heated due to current and then it glows.
Sol. If the current in the circuit is small, how can we test its presence?
Ex.25 We can test the presence of the small current by using a LED instead of the bulb.
Sol. During electrolysis of water, why does hydrogen collect on cathode and oxygen collect on anode?
Ex.26 What is the basic principle of electric pen? Explain how it can be used for writing.
Sol. Electric charge on ions is used for writing with an electric pen. Method :
(i) Take a filter paper soaked in potassium iodide solution to which a pinch of starch is added.
(ii) Place the filter paper on a metal sheet.
(iii) Connect the negative terminal of battery to the metal sheet.
(iv) Write on the paper with the end of the wire connected to the positive terminal.
(v) The writing apears on the paper. This happens because the when current is passed the K+ ions are attracted to the metal sheet. The iodide ions (I–) react with starch to turn blue black.
Ex.27 Dinesh is wants to study the chemical effect of current at home. How can he do it?
Sol. Dinesh can study the chemical effect of current at home very easily. Take two pieces of copper wire and iron wire. Place them in fresh lemon fruit. Attach the free ends of the wires to a LED. LED glows showing the flow of current.
Ex.28 Give some uses of LED. How should LED be connected?
Sol. (a) as indicators in electrical apliances.
(b) as a point source of light in laser beam torches.
(c) LEDs emitting white light can be used instead of bulbs.
Ex.29 What is the difference between current flowing through metals and current flowing through liquids?
Sol. In metals electric current is conducted by flow of electrons. in liquids, the movement of charged particles (ions) carry current from anode to cathode terminals. Electrolytes conduct current at a slow rate than metals.
EXERCISE - I
Q.1 What are conductors ?
Q.2 What are insulators ?
Q.3 Why is distilled water conductor or an insulator
Q.4 What are electrodes ?
Q.5 What is an electrolyte ?
Q.6 What happens when an electric current is passed through a conducting solution ?
Q.7 Define electroplating.
Q.8 Name one non-metal which a good conductor of electricity ?
Q.9 What is electrolysis ?
Q.10 How can distilled water be made a good conductor of electricity ?
Q.11 In what proportion the two products from electrolysis of water are obtained ?
Q.12 Name three liquids, which when tested in the manner shown in figure, may cause the magnetic needle to deflect.
Q.13 In case of a fire, before the firemen use the water hoses, they shut off the main electrical supply for the area. Explain why they do this.
Q.14 Why should you not touch electrical appliances with wet hands ?
Q.15 How are bridges and automobiles prevented from rusting ?
Q.16 Explain the process of electroplating of copper.
Q.17 During electrolysis of water, why does hydrogen collect on cathode and oxygen collect on anode ?
Q.18 Distilled water does not conduct electricity what we can do to make it conducting.
Q.19 What are anions and cations? Give examples.
Q.20 What are the use of electroplating?
Q.21 Why do we get electric shock?
Q.22 A pencil sharpened at both ends can be used as a conductor in laboratories. Why?
Q.23 What is electrical conductivity?
Q.24 We are advised to use rubber soled slippers while working with electrical goods. Why?
Q.25 What do you mean by chemical effect of curent?
Q.26 Distinguish between electrolytes and non-electrolytes. Give examples.
Q.27 What do you understand by electrolysis? Briefly explain various terms involved in the process of electrolysis.
Q.28 Briefly explain the applications of electroplating. How will you protect metals from getting corroded?
EXERCISE - II
Q.1 When electric current is passed through acidulated water, the gases produced are :
(A) Hydrogen and oxygen
(B) Hydrogen and ozone
(C) Oxygen and hydrogen peroxide
(D) None of these
Q.2 The object to be electroplated is made :
(A) Cathode (B) Anode
(C) Cathode or anode(D) Anode only
Q.3 The method of purifying metals by passing electricity is called :
(A) Electrolysis (B) Electroplating (C) Electrorefining (D) None of these
Q.4 During purification of metals, the refined metal is obtained at the :
(A) Cathode
(B) Anode
(C) Surface of electrolyte
(D) Both (A) & (B)
Q.5 During electrolysis, the electrolyte undergoes:
(A) A physical change
(B) A chemical change
(C) Either (A) or (B)
(D) None of these
Q.6 Which of the following is a non-electrolyte?
(A) Salt solution (B) Lemon juice (C) Distilled water (D) Tap water
Q.7 Which of the following is an electrolyte?
(A) Alcohol (B) Benzene
(C) Sulphuric acid (D) Kerosene oil
Q.8 Which of the following is used to carry out electrolysis?
(A) Voltmeter (B) Ammeter
(C) Voltameter (D) All of these
Q.9 In an electrolytic cell, the electrode that is connected to the positive terminal of the battery is called :
(A) Cation (B) Cathode
(C) Anion (D) Anode
Q.10 The process by which a chemical change takes place in a substance when electric current is passed through it is called :
(A) Electrolysis
(B) Electroplating
(C) Electrodes
(D) Thermionic conduction
Q.11 Adding a soluble metallic salt to water :
(A) Increases its electrical conductivity (B) Decreases its electrical conductivity
(C) Never produces any change in the conductivity
(D) None of these
Q.12 Electroplating is a method of :
(A) Making plates using electricity
(B) Plating a metal with another metal
(C) Coating any object with an electrically conducting plate
(D) Coating a metal with another metal by passing an electric current
Q.13 An electrolyte is :
(A) A light electric cell
(B) A liquid that conducts electricity
(C) A metal
(D) None of these
Q.14 A bad conductor of electricity is :
(A) Distilled water (B) Copper sulphate (C) Silver nitrate (D) Sulphuric acid
Q.15 Electroplating is not done with :
(A) Silver (B) Zinc
(C) Gold (D) Iron
Q.16 Artifical jewellery is usually coated with
(A) Gold (B) Zinc
(C) Chromium (D) Copper
Q.17 Chromium plating is done because, chromium :
(A) Is expensive
(B) Has a shiny appearance
(C) Gets corroded
(D) Does not resist scratches
Q.18 When common salt is added to distilled water, the water becomes a :
(A) Good conductor
(B) Bad conductor
(C) Moderate conductor
(D) Both (A) & (B)
Q.19 Which of the following does not involve any chemical effects of current?
(A) Electroplating (B) Electrorefining (C) Dispersion (D) None of these
Q.20 The process by which an electrolyte is decomposed with the help of electrcity is :
(A) Electroplating (B) Electrorefining (C) Electrolysis (D) None of these
Q.21 The object to be electroplated is made :
(A) Cathode
(B) Anode
(C) Cathode or anode
(D) None of these
Q.22 The method of purifying metals by passing electricity is called :
(A) Electrolysis (B) Electroplating (C) Electrorefining (D) Voltameter
Q.23 Which of the following is used to test the flow of weak electric current through a circuit?
(A) LED (B) Battery
(C) Voltameter (D) Cathode
Q.24 Negatively charged electrode is known as :
(A) Cathode (B) Anode
(C) Insulator (D) Cathode or insulator
Q.25 During purification of metals, the refined metal is obtained at the :
(A) Cathode
(B) Anode
(C) Surface of electrolyte
(D) None of these
Q.26 How many leads does an LED have?
(A) 2 (B) 3
(C) 4 (D) 8
Q.27 The longer end of LED is always connected to the ................ of the battery?
(A) Positive terminal (B) Negative terminal (C) Centre (D) Insulator
Q.28 An LED is a type of :
(A) Resistor (B) Tester
(C) Battery (D) Bulb
Q.29 Jewellers electroplate silver and gold on :
(A) Expensive metals
(B) Less expensive metals
(C) All alkali metals
(D) All of these
Q.30 Tin cans, used for storing food, are made by electroplating tin onto :
(A) Lead (B) Gold
(C) Iron (D) Chromium
ANSWERS KEYS
1. A 2. A 3. C 4. B 5. B
6. C 7. C 8. C 9. D 10. A
11. A 12. D 13. B 14. A 15. D
16. A 17. B 18. A 19. C 20. C
21. A 22. C 23. A 24. A 25. A
26. A 27. A 28. B 29. B 30. C