I. Introduction
Combustion is simply burning of a substance. It is an exothermic process, i. e., heat is produced on combustion. During combustion, carbon present in the fuel combines with oxygen and forms carbon dioxide while hydrogen forms water vapour. Therefore, the main products of combustion are CO2 and water vapour. Now the combustion may be defined as the burning of a substance in oxygen or air to produce heat and light.
The process in which a substance combines chemically with oxygen or any other supporter of combustion, with simultaneous evolution of heat and light is called combustion.
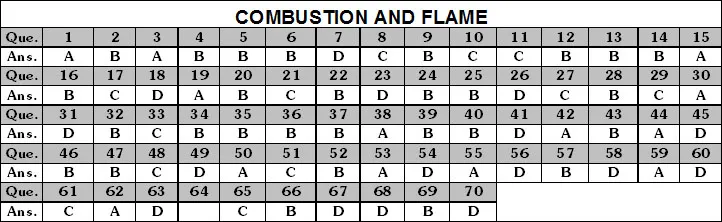
II. Rapid Combustion (or Burning)
The process in which a substance combines chemically with oxygen at a temperature above its ignition temperature with the evolution of large amounts of heat and light in a short time is called rapid combustion, or burning. Burning of hydrocarbon fuels e.g., LPG, kerosene, petrol etc., is rapid combustion.
Combustion (or burning) of some common substances are described below :
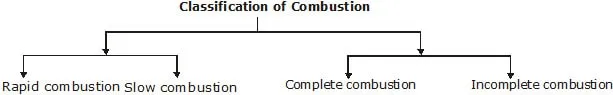
(i) Combustion of carbon :
Carbon (or charcoal) burns in air or oxygen to give CO2 producing heat and light.
![]()
(ii) Combustion of hydrocarbons : Hydrocarbons burn to produce carbon dioxide (CO2), water (H2O) and heat and light.
For example, burning of methane or natural gas is described by the equation.

Burning of LPG (which contains mainly butane) produces carbon dioxide, water, heat and light.
(iii) Combustion of magnesium : Magnesium burns in air (or oxygen) to give magnesium oxide (MgO) producing heat and light.

III. Slow Combustion
A combustion reaction in which no light is produced and temperature of the substance remains almost unchanged is called slow or spontaneous combustion.
Thus, a substance undergoes slow combustion without catching fire.
Some example of slow or spontaneous combustion are :
(i) Digestion of food (or respiration) (ii) Oxidation of yellow phosphorus at room temperature.
IV. Complete Combustion
The combustion in which the substance gets completely burnt to form the highest oxide of the substance is called complete combustion. Combustion in the presence of excess (or sufficient) oxygen or air is complete combustion.
For example, burning of carbon to carbon dioxide (CO2) is complete combustion.

V. Incomplete Combustion
The combustion reaction that takes place in the presence of insufficient quantity of oxygen (or air) is called incomplete combustion.
For example, when carbon is burnt in insufficient (limited) quantity of air, carbon monoxide is formed.
* Combustible & Non-Combustible Substances
The substances which burn readily are called combustible substances.
For example, Petrol, LPG (cooking gas), Wax, Kerosene, Paper, Cloth, Wood, Coal etc., are combustible substances.
The substances which do not burn are called non-combustible substances.
For example, Water, glass, sand etc., are non-combustible substances.
VI. Conditions Necessary for combustion :
(i) Combustible Substance :Combustible substances are the substances that can burn easily, e.g. wood, paper, cloth, petrol, kerosene, LPG, etc. Combustion of all carbon based fuels produces CO2 and H2O.
(ii) Supporter of Combustion : We know that oxygen is necessary for combustion so it is called supporter of combustion. In most of the cases, oxygen is available from air. when a burning coal is covered with a vessel, the coal fire stops. Hence, it is clear that oxygen or air is necessary for burning.
(iii) Ignition Temperature : The minimum temperature at which a substance catches fire and starts burning is known at its ignition temperature or ignition point or kindling temperature.
So, a substance must be heated to its ignition temperature so as to start burning.
Ex. The ignition temperature of a candle is low but it is higher than room temperature. So a candle does not start burning by itself at room temperature. When a burning matchstick is applied to the wick of candle, the heat produced by the matchstick raises the temperature of candle which is equal to its ignition temperature, so, the candle catches fire and starts burning. Different fuels have different ignition temperature.
FUELS
A combustible substance which on burning produces a large amount of heat and light is called a fuel. Ex. Coal, LPG , Petrol , Kerosene , wood etc.
I. Classification of Fuels
Fuels are classified on the basis of physical states in which they occur. So fuels are classified as solid, liquid and gaseous fuels.
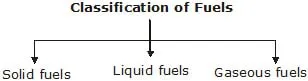
(i) Solid fuels : Combustible substances which are solid at room temperature are called solid fuels. Solid fuels contain mainly carbon both as free and combined carbon. In rural areas, Firewood, Agricultural wastes, Animal-dung cakes are the major source of energy.
Examples : Some solid fuels are :
(a) Coal (b) Coke (c) Wood (d) Charcoal
(e) Animal-dung cakes (f) Bagasse, Agricultural wastes
(ii) Liquid fuels : Volatile liquids which produce combustible vapour are called liquid fuels. Kerosene is the most commonly used liquid fuel.
Examples : Some common liquid fuels are :
(a) Petrol (b) Diesel (c) Kerosene (d) Alcohol
Petrol, diesel and kerosene are mixtures of hydrocarbons.
(iii) Gaseous fuels : Combustible gases or mixtures of combustible gases are called gaseous fuels.
Examples : Some commonly used gaseous fuels are :
(a) Natural gas (b) Liquefied petroleum gas (LPG) (c) Biogas (or Gobar gas) (d) Coal gas (e) Water gas (f) Producer gas (g) Hydrogen gas
(h) Compressed Natural (CNG) Gas
Petroleum gas is obtained as a by-product during the fractional distillation of petroleum.
II. Characteristics of an ideal fuel
An ideal fuel should have the following characteristics :
(i) It should be fairly cheap and easily available.
(ii) It should burn at moderate rate.
(iii) It should not produce any poisonous and irritating fumes during burning.
(iv) It should leave no residue (ash) after burning.
(v) It should produce large amount of heat per unit mass i.e., it should have high calorific value.
(vi) It should be safe and convenient from the storage and tranportation point of views.
(vii) Its ignition temperature should be above room temperature. So that it is safe to use such a fuel.
III. Uses of Fuels
(i) Cooking and Heating : The most common use of fuels is for cooking and heating. The commonly used domestic fuels are wood, dry cattle dung, coal, charcoal, kerosene (in rural areas) and coal, kerosene, LPG (in urban areas).
(ii) For Transportation : Fuels such as petrol, diesel and CNG are used for running cars, scooters, buses, trucks and trains. These automobiles are used for transportation from one place to another. The fuel used in aeroplanes is called aviation fuel.
(iii) For Generating Electricity : Fuels such as coal and natural gas are used for generating electricity on a commercial scale, in thermal power stations. Petrol, diesel and kerosene are also used for generating electricity in smaller generators commonly used at homes and shops, etc.
(iv) In Industry : Fuels such as coal, natural gas, diesel and furnace oil are used in the industry for generating steam in boilers. Steam is required in industry for heating purposes and also for generating electricity for their own use in factory. Industry in the rural areas also uses biomass such as bagasse—the cellulose material left after extracting juice from the sugarcane for running boilers.
(v) For Launching Space Vehicles : Space vehicles are launched with the help of rockets. Rockets use special fuels called propellants. A propellant is a combination of a fuel and an oxidizer.
FLAME
I. Introduction
A flame is a region where combustion of fuel takes place. A flame is the product of a highly exothermic reaction.
Note: Exothermic reactions are those reactions in which energy is released.
A flame is the visible (light-emitting) part of fire.
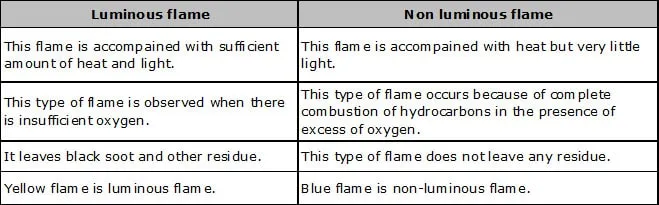
(i) Colour of Flame: Colour of flame depends on the temperature, amount of air supplied, and the substance burning.
e.g. flames produced by hydrocarbon fuels are either blue or yellow.
(ii) Types of Flame: A flame can be of two types: (a) Luminous flame (b) Non-luminous flame
II. Combustion of Wax Candle
Candles are usually made of paraffin wax, which is obtained from petroleum. When a candle is burnt, following observations can be made:
(i) The wick burns and it stands in a pool of liquid wax.
(ii) There is a small portion of unburnt whick between the flame and the liquid wax.
(iii) The liquid wax is trapped in a cup of solid wax.
(iv) The liquid or solid wax never catches fire. Also the flame never travels down to burn either liquid wax or the solid wax.
What actually happens in the process of burning of a candle?
(i) It is only the wax vapours that burn. Neither liquid wax nor solid wax burn.
(ii) When a candle wick is lit, the heat produced from the flame melts the wax.
(iii) The wick soaks or absorbs the molten wax.
(iv) The heat of the flame vapourizes the molten wax in the wick.
(v) The gaseous wax burns in the flame, which keeps the flame alive. This process goes on till the entire wax gets burnt.
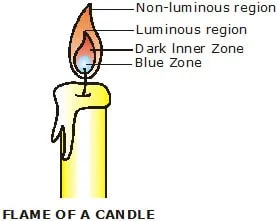
III. Structure of a Candle Flame
According to Berzelius (1822), a candle flame consists of four zones.
These are,
(i) Outermost nonluminous (blue) zone of complete combustion.
(ii) Central (or middle) luminous zone of incomplete combustion.
(iii) Inner dark zone of no combustion.
(iv) Lowest blue zone.
These zones are described below :
(i) Outermost nonluminous zone of complete combustion : This zone is faintly visible and surrounds the yellow luminous part of the flame. In this zone, the wax vapour undergoes complete combustion because plenty of air is present around it. This zone is the hottest part of the candle flame.
(ii) Central (or middle) luminous zone of incomplete combustion : The central luminous zone is the major part of the candle flame. This zone is bright yellow and luminous, and lies below the outermost nonluminous zone. In this zone, wax vapour undergoes incomplete combustion because not enough of air is present here. The incomplete combustion of wax vapour produces carbon particles. These unburnt carbon particles get heated up and start glowing. These glowing carbon particles make the flame luminous. Thus, the central zone of the candle flame is luminous due to the incomplete combustion temperature.
(iii) Inner dark zone of no combustion : The dark zone around the wick is called inner dark zone of no combustion. In this zone very little or no combustion takes place because in this zone no air is present. This zone is dark (black) due to the presence of unburnt carbon particles in the wax vapour. This part of the flame is the least hot.
(iv) The lowest blue zone : This zone is located at the base of the flame. The blue colour of this zone is due to the burning of the carbon monoxide produced in the dark zone.
SOLVED EXAMPLES
Ex.1 Why water is not suitable as fire extinguisher for fires involving oil and petrol?
Sol. As we know, water is heavier than oil so it sinks below the oil and oil keep burning on the top.
Ex.2 Define combustible substances.
Sol. The substance that can reacts with oxygen and gives off heat and light i.e., undergo combustion, is called combustible substances.
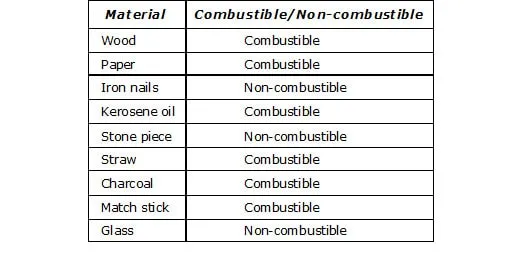
Ex.3 With the help of given table, mark the combustible and non-combustible substances of your daily life.
Sol.
Ex.4 Why we wrap a blanket around a person who caught fire?
Sol. We wrap a blanket around a person who caught fire, because the fire stops getting the oxygen in required amount for combustion and it extinguishes.
Ex.5 In what form (solid, liquid, gas) carbon dioxide be stored in cylinders?
Sol. Carbon dioxide can be stored as a liquid in cylinders.
Ex.6 With the help of an experiment, show that oxygen (air) is necessary for the burning of a candle.
Sol. To show the experiment we have to fix three lighted candle on a table, and put a glass chimney over it.
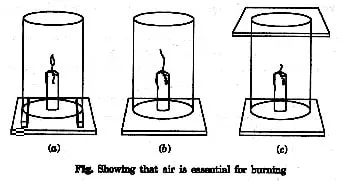
The chimney (Fig. a) rested on wooden blocks in such a way that air can enter the chimney-the candle will continue burning, because air can enter the chimney from below.
The chimney (Fig. b) rested on the table with no way for air-the flame flickers and produces smoke.
The chimney (Fig. c) rested on table and covered from top with glass plate-the flame goes off because the air is not available.
Ex.7 What do you mean by inflammable substances?
Sol. The substance which have very low ignition temperature and can easily catch fire with a flame are called inflammable substances.
Ex.8 What is rapid combustion?
Sol. A combustion that takes place at a high speed is known as rapid combustion.
Ex.9 What is spontaneous burning?
Sol. In a heap of several substances, when they catches fire, they undergo slow oxidation with evolution of heat. At certain stage when the temperature becomes more than the ignition temperature of anyone of the constituent-all of a sudden, it starts burning. This is called spontaneous burning.
Ex.10 Give the characteristics of Ideal fuel?
Sol. 1. It is readily available. 2. It is cheap.
3. It burns easily at moderate rate. 4. It produces a large amount of heat.
5. It does not leave behind any undesirable substances.
Ex.11 Charcoal does not burn with flame but glows only.
Sol. Charcoal does not burn with flame because it is not volatile in nature or does not vapourise on burning, so it glows only.
Ex.12 What is meant by the term 'fuel'?
Sol. A fuel is a substance, which may be burnt to produce considerable heat without the formation of undesirable products.
Ex.13 Why we are advised never to sleep in a room with burning coal fire in it?
Sol. The carbon monoxide gas produced by incomplete combustion of coal can kill persons sleeping in that room because it is a poisonous gas.
Ex.14 What are the constituents of a foam type fire extinguisher? 
Sol. The foam type fire extinguisher is also called as 'Soda acid' fire extinguisher.
The constituents are:
(a) Dilute sulphuric acid (b) Sodium bicarbonate (soda).
When it is operated, it comes out under pressure in the form of a foam,
which covers the surface of fire and controls it.
Ex.16 Describe the various zones of a flame.
Sol. A flame has four zones in it:
(a) The outermost thin transparent faint bluish nonluminous region of complete combustion. It is the hottest zone of the flame. 
(b) The middle bright luminous zone of partial combustion. It is the moderately hot zone.
(c) The innermost, coldest dark zone, consists of hot vapour and
called as zone of no combustion.
(d) Blue zone near the bottom of the flame is due to burning of CO.
CO is formed due to incomplete combustion.
Ex.17 What are the different harmful products formed by the burning of a fuel?
Sol. The harmful product formed by the burning of a fuel:
1. Carbon fuels release unburnt carbon particles. These fine particles are dangerous pollutants causes respiratory problems.
2. Incomplete combustion of carbon fuels gives carbon mono oxide a poisonous gas that can even kill a person sleeping in a closed room.
Ex.18 Explain the term "global warming".
Sol. The combustion of fuels release carbon dioxide in the environment. When the percentage of carbon dioxide increases in the air and makes the earth surface hot-it is believed to cause global warming.
Ex.19 What is acid rain?
Sol. The gases like nitrogen oxide, sulphur dioxide etc. present in the atmosphere as pollutants when these gases dissolve in rain water and form acids and reaches to the earth as rain called as acid rain.
Ex.20 Why CNG is preferable for vehicles in respect to petrol and diesel?
Sol. CNG (compressed natural gas) is preferable for vehicles in respect to petrol and diesel, because:
(a) It leaves least residues. (b) It is cheaper. (c) It is eco-friendly.
Ex.21 Why any of the fuel is not considered as an ideal fuel?
Sol. The fuel is not considered as an ideal fuel because all the fuels are differ in their cost and efficiency, i.e., its calorific value.
Ex.22 "Food is a fuel for our body". Explain how?
Sol. In our body, food is broken down by reaction with oxygen and heat is produced that is why food is a type of fuel for our body.
Ex.23 What chemicals are used in the preparation of matchsticks?
Sol. The head of safety match contains only antimony trisulphide and potassium chlorate.
Ex.24 Which have higher ignition temperature - kerosene oil or wood.
Sol. The kerosene oil has lower ignition temperature than wood.
Ex.25 What are the three essential requirements for producing fire?
Sol. The three essential requirements are
(i) Fuel (ii) Air (to supply oxygen)
(iii) Heat (to raise the temperature of the fuel beyond the ignition temperature).
Ex.26 List conditions under which combustion can take place.
Sol. There are three essential conditions of combustion:
(a) Presence of a combustible substance.
(b) Presence of oxygen, i.e., supporter of combustion.
(c) Attainment of ignition temperature.
Ex.27 Fill in the blanks:
(a) Burning of wood and coal causes ................. of air.
(b) A liquid fuel, used in homes is .................
(c) Fuel must be heated to its ................. before it starts burning.
(d) Fire produced by oil cannot be controlled by .................
Sol. (a) Pollution (b) LPG (c) Ignition temperature (d) Water.
Ex.28 Explain how the use of CNG in automobiles has reduced pollution in our cities.
Sol. CNG is cheap, readily available and highly combustible. It has high calorific value.
It does not produce gases or residues when used in automobiles, so it reduced the pollution in our cities.
The use of CNG in automobiles has reduced pollution in our cities because CNG produces the harmful product in very small amount and it is a cleaner fuel.
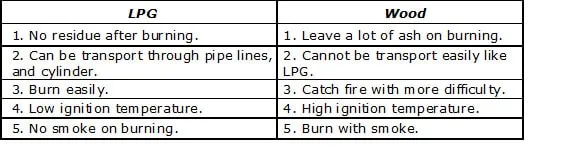
Ex.29 Compare LPG and wood as fuels.
Give reasons:
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Sol. (a) Water is not used to control the fire involving electric equipment because it is a good conductor of electricity and harm those trying to douse the fire.
(b) LPG is better domestic fuel than wood because it neither produce gases nor residues that pollute the environment.
(c) The paper by itself catches fire easily because its ignition temperature is low, while a piece of paper wrapped around an aluminium pipe does not catches fire, because its ignition temperature rises.
Ex.30 Make a labelled diagram of a candle flame.
Sol. 
Ex.31 Name the unit in which the calorific value of a fuel is expressed.
Sol. The calorific value of a fuel is expressed in a unit called kilojoule per kg (kJ/kg).
Ex.32 Explain how CO2 is able to control fires.
Sol. CO2, being heavier than oxygen, covers the fire like blanket and also brings down the temperature of fuel. Since the contact between the fuel and oxygen is cut off, the fire is controlled.
Ex.33 It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Sol. To burn a heap of green leaves is difficult, because its ignition temperature is high, but dry leaves catch fire easily as its ignition temperature switches to low.
Ex.34 Which zone of a flame does a goldsmith use for melting gold and silver and why?
Sol. A goldsmith uses the outermost zone of a flame for melting gold and silver because it is the hottest zone of the flame (temperature"" 800°C) and is non-luminous in nature.
Ex.35 In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Sol. The calorific value of the fuel:
Calorific value = kJ/kg = 180,000/4.5 kJ/kg = 40,000 kJ/kg.
EXERCISE -I (UNSOLVED PROBLEMS)
Q.1 A circular blackish ring of unburnt particles are present in ........ zone.
Q.2 Define combustion.
Q.3 Write a difference between burning of a candle and burning of coal.
Q.4 What do you understand by combustible substances or fuels ?
Q.5 Is burning of magnesium combustion ?
Q.6 Give two examples of non-combustible substances.
Q.7 What is essential for combustion ?
Q.8 What do you mean by ignition temperature?
Q.9 Does a matchstick burn by itself ?
Q.10 What is the compsition of the head of the matchstick ?
Q.11 Which type of pollution occurs on burning wood ?
Q.12 When a burning charcoal piece is covered with a glass jar then burning of the piece stops, why ?
Q.13 Which will get fire first coal or kerosene ?
Q.14 Which is the most common fire extinguisher?
Q.15 Which poisonous gas is produced due to incomplete combustion of a fuel ?
Q.16 Name the substance used to extinguish fire involving electrical equipments.
Q.17 What are inflammable substances ?
Q.18 What would you do when the clothes of a person catch fire ?
Q.19 How is CO2 able to control fire ?
Q.20 Which zone of a flame does a goldsmith use for melting gold and silver and why ?
Q.21 What are the three zones of a flame ? Draw a labelled diagram of a candle flame.
Q.22 Why does the matchstick start burning on rubbing it on the side of the matchbox ?
Q.23 Define –
(a) Spontaneous combustion.
(b) Rapid combustion.
Q.24 What are the essential requirements for producing fire ? On which principle the fire extinguisher works ?
Q.25 (i) What is calorific value ? Write its unit.
(ii) In an experiment 4.5 kg of a fuel was completely burnt.
The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Q.26 Why is it difficult to burn a heap of green leaves but dry leaves catch fire easily ?
Q.27 What are the characteristics of an ideal fuel?
Q.28 Why is CO2 an excellent fire extinguisher ? Draw a diagram of fire extinguisher.
Q.29 Name the petroleum product used for making candles.
Q.30 What is a flame? List the differences between luminous and non-luminous flames.
Q.31 Give two examples each for solid fuels, liquid fuels, gaseous fuels, and inflammable substances.
Q.32 Give two examples of non-renewable sources of energy.
Q.33 Give two examples of renewable sources of energy. Explain why they are considered renewable.
Q.34 How are fules classified?Explain with examples
EXERCISE -II
Q.1 Which of the following is not the attribute of a good fuel?
(A) Low calorific value
(B) Moderate rate of combustion
(C) Fairly cheap and easily available
(D) Safe to handle, store, and transport
Q.2 Calorific value of a fuel may be defined as :
(A) The amount of heat produced when 1 kg of a fuel is completely burnt
(B) The amount of heat produced when 1g of a fuel is completely burnt
(C) The amount of heat produced when 10 g of a fuel is completely burnt
(D) The amount of heat produced in kilojoules when 1 g of a fuel is completely burnt
Q.3 Which of the following fuels has the highest calorific value?
(A) Petrol (B) Hydrogen
(C) LPG (D) Natural gas
Q.4 In which zone of a candle flame does complete combustion take place?
(A) Inner (B) Outer
(C) Middle (D) All three zones
Q.5 Which of the following is not a necessary condition of combustion?
(A) Presence of combustible substance
(B) Presence of a supporter of combustion
(C) Attainment of ignition temperature of the fuel
(D) Presence of carbon dioxide.
Q.6 A luminous flame appears :
(A) Red (B) Green
(C) Yellow (D) Blue
Q.7 Which one of the following is a non-combustible substance?
(A) Coal (B) Iron
(C) Straw (D) Wood
Q.8 Which zone is hotter than luminous zone?
(A) Non-luminous zone
(B) Dark zone
(C) Blue zone
(D) None of these
Q.9 Water cannot be used in :
(A) Oil fire (B) Forest fire
(C) Building fire (D) Electrical fire
Q.10 Which is a zone of no combustion?
(A) Blue zone (B) Dark zone
(C) Luminous zone (D) All of the above
Q.11 Which is not used as a fire extinguisher?
(A) Water (B) Carbon dioxide (C) Oxygen (D) All of the above
Q.12 Glass is :
(A) Non-combustible (B) Combustible (C) None of these (D) All of the above
Q.13 Camphor
(A) Vaporizes in air (B) Condenses in air (C) Both (A) & (B) (D) None of these
Q.14 Carbon monoxide burns with :
(A) No flame (B) Yellow flame (C) Blue flame (D) All of the above
Q.15 For combustion reactions :
(A) Air is essential
(B) Combustible substance is essential
(C) Both (A) & (B)
(D) None of these
Q.16 Dark zone is :
(A) Visible (B) Invisible
(C) Green in colour (D) None of thesere fuels classified? Explain with examples.
ANSWERS KEY
1. A 2. A 3. B 4. B
5. D 6. C 7. B 8. C
9. D 10. B 11. C 12. A
13. A 14. B 15. C 16. B
EXERCISE III
1. Hydrocarbon burns to produce -
(A) CO2 + Water vapour + Heat + Light (B) CO + O2
(C) CO2 + H2 + Engery (D) CO + H2
2. Water cannot be used as fire extinguisher to put out-
(A) Burning wood (B) Burning oil (C) Burning cloth (D) Burning charcoal
3. In soda-acid fire extinguisher : 2NaHCO3 + 2H2SO4 ® ?
(A) Na2SO4 + 2H2O + 2CO2 (B) Na2SO4 + 2H2O + 2CO2
(C) Na2SO4 + H2O + O2 (D) Na2SO4 + H2O2
4. In soda-acid fire extinguisher
(A) CO2 (B) CO2 + H2O (C) H2O (D) H2O + H2
5. Type of flame produced by a fuel depends upon -
(A) Calorific value (B) Amount of oxygen
(C) Type of fuel and its chemical composition (D) All of the above
6. Which of the following statement is NOT true -
(A) CO2 is the best fire extinguisher
(B) Global warming can cause acid rain
(C) Burning of coal and diesel releases sulphur dioxide gas
(D) LPG has higher calorific value than biogas
7. In foam type fire extinguisher, which of the following products are formed -
Al2(SO4)3 + 6NaHCO3 ® ?
(A) 2Al(OH)3 + 6H2O + 3NaSO4 (B) 2Al(OH)3 + 6CO + 3NaSO4
(C) 2Al(OH)3 + 6O2 + 3NaSO4 (D) 2Al(OH)3 + 6CO2 + 3Na2SO4
8. In foam type fire extinguisher is responsible to put off fire -
(A) H2O (B) CO2 + H2O (C) CO2 (D) CO
9. Which of the followings is an ideal fuel -
(A) Coal (B) LPG (C) Petrol (D) None of these
10. Match column 'A' with column 'B' as regard to characteristic of a flame and its zone.
a. Dark inner zone 1. Hottest part (No carbon)
b. Bule zone 2. Partial decomposition
c. Luminous zone 3. Unburnt vapours of wax
d. Non-luminous zone 4. Complete combustion
(A) a-1, b-2, c-3, d-4 (B) a-2, b-3, c-4, d-1
(C) a-3, b-4, c-2, d-1 (D) a-4, b-1, c-2, d-3
11. Which of the following statement is false -
(A) Fossil fuels are preserved in the earth's curst
(B) A good fuel should have high calorific value
(C) Oxygen is a combustible gas
(D) A substance burns in air, if its temperature is above the ignition temperature
12. In destructive distillation, coal is heated strongly to -
(A) 5000°C (B) 1000°C (C) 100°C (D) 10,000°C
13. Wax is a -
(A) Only carbon compound (B) Hydrocarbon
(C) Chlorinated hydrocarbon (D) None of these
14. Calorific value of a fuel is the heat energy produced when-
(A) One gram of the fuel is completely burnt
(B) One kilogram of the fuel is completely burnt
(C) One miligram of the fuel is completely burnt
(D) Hundred grams of the fuel are completely burnt
15. Which type of fire extinguisher is used to extinguish fire cause by burning oil and petrol-
(A) Foam type (B) Water type (C) Soda acid type (D) CCl4 type
16. In foam type extinguisher ..........is present in bottle -
(A) NaHCO3 (B) Al2(SO4)3 (C) Al(OH)3 (D) Na2SO4
17. L.P.G. used as domestic fuel is mixed with a strong smelling volatile liquid for-
(A) Better combustion (B) Good smell/fragrance
(C) Detection of gas leakage (D) Higher calorific value
18. Hydrogen gas has the highest calorific value i.e. 150kJ/kg. yet it is not used as a fuel. The reasons are-
(A) It is explosive in nature (B) It causes storage leakage
(C) It causes transportation problem (D) All of the above
19. In foam type extinguisher present in the cylinder-
(A) Al2(SO4)3 (B) NaHCO3 (C) Na2SO4 (D) Al(OH)3
20. In soda-acid extinguisher, the bottle contains-
(A) H2so4 (B) NaHCO3 (C) NaOH (D) HCl
21. The metallic cylinder in soda-acid extinguisher contains -
(A) NaOH (B) NaHCO3 (C) Al(OH)3 (D) KOH
22. The type of combustion in which heat light and sound are produced is-
(A) Rapid (B) Explosive (C) Spontaneous (D) None of these
23. Producer gas is not a good fuel because-
(A) It contains CO which is poisonous (B) It contains CO2
(C) It contains CO2 which does not burnt
(D) It contains N2 which does not burn
24. The zone of flame having maximum temperature is-
(A) Luminous (B) Non-luminous (C) Dark zone (D) None of these
25. In L.P.G. cylinder, the gas is liquefied by -
(A) Increasing volume (B) Applying pressure
(C) Increasing temperature (D) Replacing pressure
26. Which among the following is the best fuel -
(A) Wood 4000 Cal/g (B) Wood 8000 Cal/g
(C) Wood 7000 Cal/g (D) Kerosene 11,200 Cal/g
27. Which of the following has the highest calorific value -
(A) Petrol (B) Coke (C) Natural gas (D) Kerosene
28. Which of the following has the characteristics of of a good fuel -
(A) Coke (B) Butane (C) Coal (D) Kerosene
29. The process of burning a substance in the presence of air with the evolution of heat is called -
(A) Distillation (B) Carbonisation (C) Combustion (D) Refining
30. Methane burns in air to form -
(A) CO2 + H2O (B) CO2 + H2 (C) CO + O2 (D) CO2 + O2 + heat
31. Combustion is a ...............reaction accompainied by heat and light -
(A) Reduction (B) Redox (C) Substitution (D) Oxidation
32. Types of combustion are-
(A) Rapid (B) Spontaneous, Rapid, Slow
(C) Explosive (D) Spontaneous
33. The process of burning a substance in the presence of air with evolution of heat is called -
(A) Distillation (B) Carbonisation (C) Combustion (D) Refining
34. The minimum temperature at which a substance catches fire is called -
(A) Boiling temperature (B) Ignition temperature
(C) Melting temperature (D) None of these
35. Incomplete combustion of methane forms -
(A) CO2 + H2O (B) CO + H2 (C) CO2 + H2 (D) CO + O2
36. Which of the following is an example of rapid combustion -
(A) Candle (B) Cracker (C) White phosphorous (D) None
37. The substances have high ignition temperature -
(A) Need low temperature to burn (B) Need high temperature to burn
(C) Can burn any temperature (D) Always show exothermic reactions
. The central zone of the candle flame is luminous because of -
38 (A) Incomplete combustion of wax vapour (B) Complete combustion of wax vapour
(C) No combustion of wax vapour (D) None of these
39. An ideal fuel should -
(A) Leave ash after burning (B) Have ignition temp above roo temp
40. (C) Produce poisonous and irritating fumes (D) None of these
Which of the following is a noncombustible substances-
(A) Coke (B) Diamond (C) Coal (D) Wood
41. The lowest temperature at which a substances starts burning is called -
(A) Minimum temperature (B) Maximum temperature
(C) Boiling temperature (D) Ignition temperature
42. A burning substances will be .........if the temperature falls below its ignition temperature-
(A) Extinguished (B) Burning brightly (C) Burning dimly (D) burning with smoke
43. Types of combustion are-
(A) Rapid (B) Spontaneous, rapid and explosive
(C) Explosive (D) Spontaneous
44. Which of the following is an example of rapid combustion -
(A) Candle (B) Cracker (C) White phosphorus (D) None
45. Fire extinguished by -
(A) Removing all combustible substance (B) Cutting off supply of air
(C) Cooling the burning substance (D) All of these
46. LPG is mainly a mixture of two hydrocarbons-
(A) Butane + ethane (B) Butane + isobutane (C) Propane + ethane (D) Methane + ethane
47. The heat produced by burning of 1g of fuel completely is known as -
(A) Heat capacity (B) Calorific value (C) Vapour density (D) Boiling point
48. For a spontaneous combustion to take place -
(A) Flame is required (B) Spark is replied
(C) Rise in temp to ignition temp is required (D) Nothing is required
49. An electric spark is struck between two electrodes placed near each other, inside a closed tank full of petrol. What will happen-
(A) Spontaneous combustion of petrol (B) Explosion
(C) Slow combustion of petrol (D) Nothing will happen
50. Natural gas consists of -
(A) Methane,ethane, propane and ethylene (B) Methane,propane and ethane
(C) Pantene, methane and ethylene (D) Hexane, butane and ethane.
51. Synthetic petroleum is artificially produced from -
(A) Petroleum (B) Wood (C) Coal (D) L.P.G.
52. Arrange the following fuels in increasing order of their calorific value-
1. Petrol 2. Diesel 3. Kerosene 4. Natural gas
(A) 1, 2, 3, 4 (B) 2, 3, 4, 1 (C) 2, 1, 3, 4 (D) 2, 3, 1, 4
53. Incomplete combustion of fuel gives -
(A) CO (B) CO2 (C) SO2 (D) Oxides of nitrogen
54. The nature of flame depends on -
(A) Ignition temperature (B) Chemical nature of combustible material
(C) Appratus used for burning (D) All of above
55. Which of the following is hottest zone of a candle flame -
(A) Non-luminous zone (B) Luminous zone
(C) Invisible zone (D) All zone produce equal light
56. The condition necessary for combustion is -
(A) Presence of combustion substances (B) Presence of supporter of combustion
(C) Attainment of Ignition temperature (D) All of these
57. Water cannot be used as fire extinguisher to put out
(A) Burning wood (B) Burning oil (C) Burning cloth (D) Burning charcoal
58. Total amount of heat produced by a fuel having calorific value of 20 kJ/kg was found to be 50,000 Joules. How much fuel was burnt ?
(A) 2500 kg (B) 250 kg (C) 25 kg (D) 2.5 kg
59. Combustion is an :
(A) Exothermic reaction (B) Endothermic reaction
(C) Displacement reaction (D) Reduction reaction
60. Inspite of danger involved with hydrogen, it, used as fuel for some applications. What are these ?
(A) Rocket fuel (B) Oxyhydrogen flame
(C) Car fuel (D) All of the above
61. Green house effect is caused due to trapping of :
(A) Ultraviolet radiations (B) Microwave radiations
(C) Infra-red radiations (D) Cosmic radiations
62. Carbon monoxide combines with haemoglobin in the blood to form :
(A) Carboxy haemoglobin (B) Carbonic acid
(C) Both (A) and (B) (D) None of the above
63. Whih statement is incorrect ?
(A) Hydrogen is having highest calorific value
(B) Storage of CNG is difficult
(C) When we use coal as a fuel, the ash is formed as a residue
(D) SPM ans SO2 are released by petrol & kerosene
64. If the clothes of a person catch fire, the person is immediately wrapped in a thick blanket then :
(A) The fire goes off (B) The fire intensifies
(C) It burns with same pace (D) None of these
65. A family consumes 12 kg of LPG in 30 days. Calculate the average energy consumed perday if the calorific value of LPG is 50 kJ/kg.
(A) 10,000 J/day (B) 15,000 J/day (C) 20,000 J/day (D) 25,000 J/day
66. Inflammable substances have an ignition temperature :
(A) More then 100°C (B) Less than 100°C
(C) More than 200°C (D) Between 200°C to 350°C
67. The disadvantages of incomplete combustion is / are :
(A) Unburnt carbon gets released (B) Air is polluted
(C) May cause respiratory problems (D) All of the above
68. The blue zone in a LPG flame indicates :
(A) Unburnt vapours (B) Partial decomposition
(C) Moderately hot (D) Hottest and complete combustion
69. Which of the following is an example of rapid combustion ?
(A) Burning of wood (B) Burning of LPG (C) Burning of cracker (D) Burning of a candle
70. Acid rain is caused by :
(A) Deforestation (B) CO2
(C) CO (D) Oxides of sulphur and nitrogen