Lavoiser classified all elements into metals, nonmetals and metalloids on the basis of their properties. Some commonly used metals, nonmetals and metalloids are given below.
(i) Metals : Iron, Copper, Gold, Silver, Aluminium, Zinc, Lead are some commonly used metals.
(ii) Nonmetals : Hydrogen, Oxygen, Nitrogen, Carbon, Sulphur, Phosphorus, Chlorine, Bromine, Iodine are commonly used nonmetals.
(iii) Metalloids : Boron, Silicon, Arsenic and Germanium are some metalloids.
CHARACTERISTICS OF METALS
Some important characteristics of metals are :
(i) Metals are good conductors of heat and electricity.
(ii) All metals except mercury are solid at room temperature. Mercury is the only metal which is liquid at room temperature.
(iii) Metals are malleable and ductile – that is metals can be beaten into thin sheets and drawn into thin wires.
(iv) Metals have lustre and can be polished.
(v) Metals have tensile strength.
(vi) Metals are electropositive elements. That is, metals have a tendency to lose electrons and form positively charged ions, (called cations).
I. Occurrence of Metals
Metals occur in nature in the free as well as in the combined states.
(i) All metals which are not affected by water and by the gases present in the air occur in free state in nature.
(ii) The naturally-occurring compounds of metals mixed with earthly materials are called minerals.
(iii) A mineral from which a metal can be extracted on the commercial scale, economically and easily, is called an ore.
II. Physical Properties of Metals
All metals show similar physical properties. There are however a few exceptions.
(i) Physical State : Under normal pressure, all metals except mercury are solids at room temperature. Mercury is liquid at room temperature.
(ii) Colour : Most metals except gold and copper are silver-grey in colour. Copper is reddish-brown and gold is golden yellow.
(iii) Appearance : All metals are shiny. The characteristic shine of metals is called metallic lustre. Thus all metals have metallic lustre. Metals can be easily polished.
(iv) Hardness : Most metals are hard except sodium and potassium. Sodium and potassium metals can be easily cut with a knife. Osmium is hard enough to scratch glass.
(v) Tensile strength : Metals have high tensile strength. Metals are very strong. For example, iron can bear a lot of stress. That is why it is widely used in construction of buildings, bridges, railway lines etc.
(vi) Malleability : Metals are malleable. This means that metals can be hammered into very thin sheets. Silver can be beaten to very thin leaves. You must have seen silver varak on burfee. Aluminium foil is used in the packaging of food materials.
(vii) Ductility : Metals are ductile. This means that metals can be drawn into thin wires. Silver and gold can be drawn into very thin wires.
(viii) Conductivity : Metals are good conductor of heat and electricity. Silver is the best conductor of electricity. Copper is the next best conductor of electricity.
(ix) Density : Metals, except sodium and potassium have high densities. Sodium and potassium have much lower densities.
(x) Sound : Metals are sonorous. Metals when hit by a hammer produce a ringing sound. That is why metal are used for making bells and wires for musical instruments.
CHEMICAL PROPERTIES OF METALS
All metals give similar chemical reactions. However, the reactivity of a metal depends upon its nature and reaction conditions.
Some typical reactions of metals are described below :
(i) Reaction with oxygen : All metals combine with oxygen to form metal oxides. Different metals react with oxygen under different conditions.
For example,
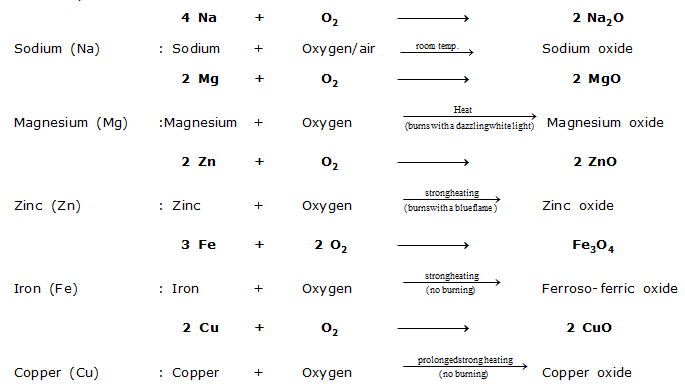
From the reaction conditions of the reactions given above, the order of reactivity of metals with oxygen is,
Sodium (Na) > Magnesium (Mg) > Zinc (Zn) > Iron (Fe) > Copper (Cu)
(ii) Reaction with Water : Different metals react with water under different conditions.
Reactions of some common metals with water are given below :
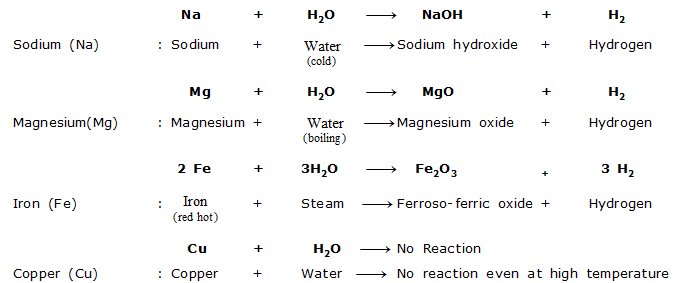
From the reaction conditions of the above reactions, the order of reactivity of metals with water is
Sodium (Na) > Magnesium (Mg) > Iron (Fe) > Copper (Cu)
(iii) Reactions with Acids : Most metals react with dilute acids produce salt and hydrogen gas.
Reaction of some common metals with dilute hydrochloric acid are given below :
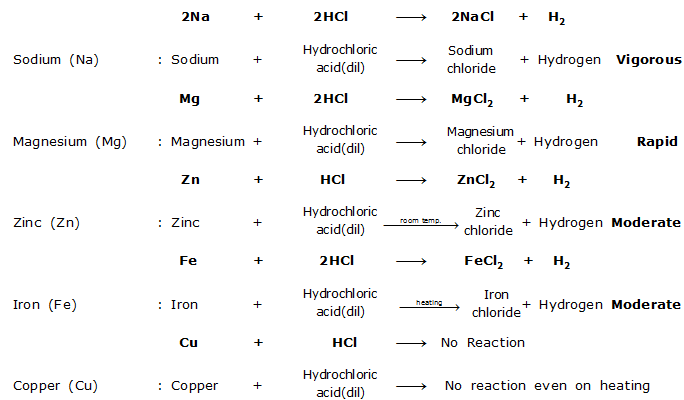
From the reaction conditions of the reaction given above, the order of reactivity of these metals with dilute acid is
Sodium (Na) > Magnesium (Mg) > Zinc (Zn) > Iron (Fe) > Copper (Cu)
Uses of some Common Metals
Main uses of some common metals are listed below :
CHARACTERISTICS OF NON METALS
Some important characteristics of metals are :
(i) Nonmetals are soft solids, liquids or gases.
(ii) Nonmetals (except graphite) are nonconductors of heat and electricity.
(iii) Solid nonmetals are brittle.
(iv) Nonmetals (except graphite and diamond) are low melting and low boiling.
(v) Nonmetals are electronegative elements. That is, nonmetals have a tendency to gain electrons and form negatively charged ions (called anions).
III. Occurrence of Nonmetals
Many nonmetals occur free in nature, whereas many more occur only in the form on their compounds as minerals.
The modes of occurrence of some typical nonmetal are described below :
Most nonmetals are either mined directly from their mines or obtained as by-products in some industrial processes.
(i) Nitrogen and Oxygen are obtained from the air by fractional distillation of liquid air.
(ii) Chlorine is obtained from common salt by electrolytic method.
(iii) Sulphur is mined in its elemental form
(iv) Nonmetals such as phosphorus and silica are obtained from their ores by chemical methods.
IV. Physical Properties of Nonmetals
Some common general physical properties of nonmetals are given below :
(i) Physical state : Nonmetals may occur as solids, liquids or gases at room temperature.
For example, under normal conditions, sulphur, phosphorus are solids, bromine is a liquid, whereas hydrogen, oxygen and nitrogen are gases.
(ii) Colour : Nonmetals come in many colours.
For example, sulphur is yellow, phosphorus is white, or red, chlorine is greenish-yellow, bromine is redish-brown. Hydrogen, oxygen and nitrogen are colourless.
(iii) Appearance : Nonmetals have dull appearance i.e., they do not shine. However, graphite and iodine are the only nonmetals which have metallic lustre.
(iv) Malleability and ductility : Nonmetals are neither ductile nor malleable. Nonmetals cannot be drawn into wires, and beaten into leaves/sheets.
(v) Conductivity : Nonmetals do not conduct heat and electricity, i.e., nonmetals are insulators. Graphite however, is a good conductor of heat and electricity.
(vi) Density : Nonmetals usually have low densities and are soft. Diamond however is an exception. Diamond is the hardest natural substance known.
(vii) Tensile strength : Nonmetals have low tensile strength, i.e., Nonmetals can be easily broken.
(ix) Melting and boiling points : Nonmetals except graphite have low melting and boiling points.
(x) Sound : Nonmetals do not produce sound when hit with an object, i.e., nonmetals are non-sonorous.
CHEMICAL PROPERTIES OF NO
Some general chemical properties of nonmetals are described below :
V. Electronegative Character
Nonmetals are electronegative elements. Nonmetals have a tendency to accept electrons and form negatively charged ions (anions).
For examples.
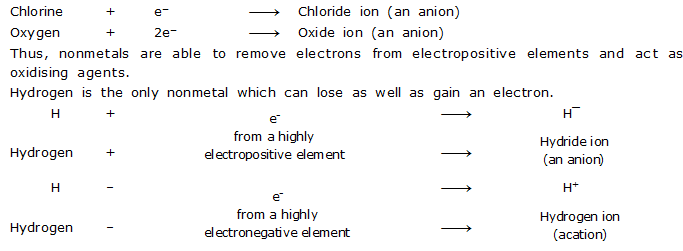
Thus, hydrogen can act both as an oxidising as well as reducing agent.
VI. Reaction with Oxygen
Nonmetals react with oxygen to give covalent oxides. Such oxides are either neutral or acidic in nature. Acids oxides of nonmetals dissolve in water to form corresponding acids. Reaction of some common nonmetals with oxygen are described below:
(i) Nitrogen : Nitrogen reacts with oxygen under different conditions to form five different oxides. Some of these are neutral, while others are acidic in nature.
For example,
Nitrogen + Oxygen ---> Nitrous oxide (neutral)
Nitrogen + Oxygen ---> Nitric oxide (neutral)
Nitrogen + Oxygen ---> Dinitrogen trioxide (acidic)
Nitrogen + Oxygen ---> Nitrogen dioxide (acidic)
Nitrogen + Oxygen ---> Dinitrogen pentoxide (acidic)
Dinitrogen pentoxide reacts with water to give nitric acid.
Dinitrogen pentoxide + Water ---> Nitric acid.
(ii) Carbon : Carbon reacts with oxygen to form two oxides – carbon monoxide (CO) and carbon dioxide (CO2). Carbon monoxide is neutral, whereas carbon dioxide (CO2) is acidic in nature. Carbon dioxide dissolves in water to give carbonic acid.
Carbon + Oxygen(limited supply) ---> carbon monoxide (neutral) (CO)
Carbon + Oxygen(excess supply) ---> carbon diaoxide (acidic) (CO2)
Carbon dioxide + Water --- > Carbonic acid (H2CO3)
(iii) Phosphorus : Phosphorus reacts with oxygen to give two oxides – phosphorus trioxide (P2O3) and phosphorus pentoxide (P2O5). Both are acidic oxides.
Phosphorus + Oxygen(limited) ----> Phosphorus trioxide (P2O3)
Phosphorus + Oxygen(excess) ----> Phosphorus pentaoxide (P2O5)
(iv) Sulphur : Sulphur on burning in air forms two oxides – sulphur dioxide (SO2) and sulphur trioxide (SO3). Both these oxides are acidic.
S + O2 ----> SO2
Sulphur + Oxygen ----> Sulphur diaoxide (acidic)
S + O2 ----> SO3
Sulphur + Oxygen ----> Sulphur trioxide (acidic)
SO3 + H2O ----> H2SO4
Sulphur trioxide + Water ----> Sulphuric acid
(v) Hydrogen : Hydrogen reacts with oxygen to form an oxide H2O. H2O is called water. Water (H2O) is a neutral oxide
H2 + O2 ---> H2O
Hydrogen + Oxygen ----> Water (neutral)
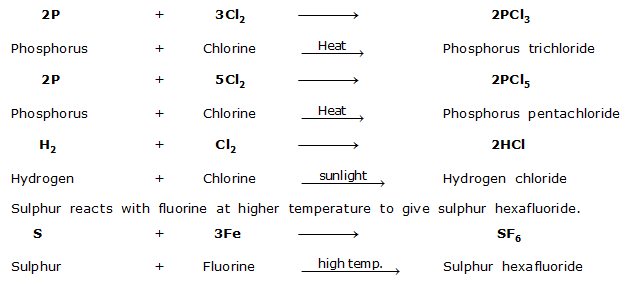
VII. Reaction with Halogens
Nonmetals react with halogen to give covalent halides. In pure state, the halides of nonmetals do not conduct electricity.
For example with chlorine,
VIII. Reaction with Hydrogen
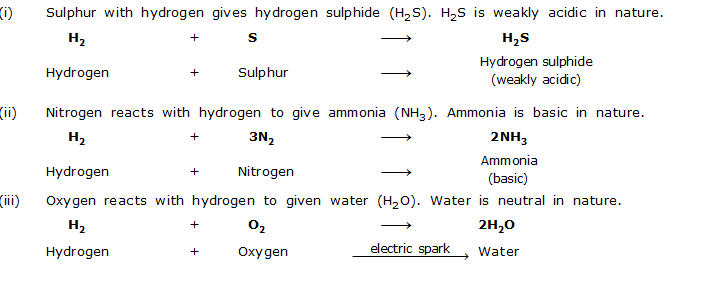
Nonmetals react with hydrogen to form covalent hydrides. Thus in the hydrides of nonmetals, hydrogen is bonded to the nonmetal atom by covalent bonds. The hydrides of nonmetals atom by covalent bonds. The hydrides of nonmetals do not conduct electricity. The hydrides of nonmetals may be acidic, basic or neutral depending upon the nature of the nonmetal.
For example,
IX. Reaction with Acids
Nonmetals do not displace hydrogen from dilute acids. This is because nonmetals are able to give electron(s) for the reduction of H+. Some nonmetals however react with concentrated oxidising acids to form the corresponding oxyacids.
For example, sulphur reacts with conc. nitric acid to give sulphuric acid.

X. Displacement Reactions
Certain more reactive nonmetals displace less reactive nonmetals from their salt solutions.
For example, Chlorine displaces bromine from bromides and iodine from iodides.

Uses of Some Common Nonmetals
Main uses of some common nonmetals are listed below :
Oxides of Metals and Nonmetals
Both metals and nonmetals react with oxygen (present in the air) to form oxides. The oxides of metals and nonmetals differ in their properties.
XI. Oxides of Metals
The oxides of metals are basic in nature. When dissolved in water, metal oxides give alkaline (or basic) solution which turn red litmus blue.
For example, magnesium (Mg) burns in air to give magnesium oxide (MgO), which is basic in nature.
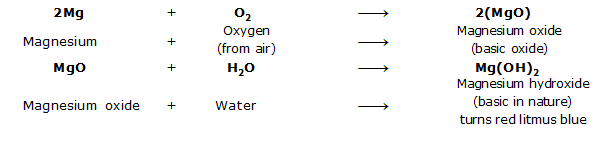
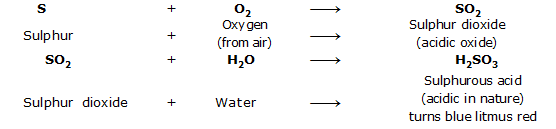
XII. Oxides of Nonmetals
The oxides of nonmetals are acidic in nature. When dissolved in water nonmetal oxides give acidic give solutions which turn blue litmus red.
For example, sulphur on burning in air, gives sulphur dioxide (SO2) which is acidic in nature.
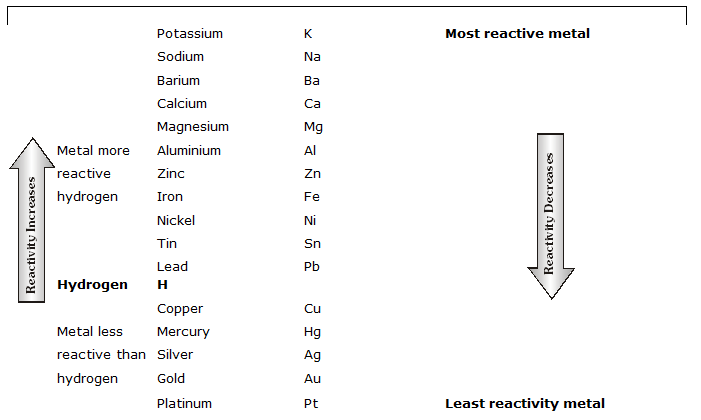
REACTIVITY SERIES OF METALS
(a) The more reactive metals always displace less reactive metals in chemical reactions. If a metal loses electrons easily to form positive ions, it will readily react with other substances. On the other hand if a metal loses electrons less rapidly to form a positive ion, it will react slowly with the other substances. Such a metal will be less reactive.
"The arrangement of metals in the order of decreasing reactivities is called the reactivity series or activity series of metals".
(b) Utility of Activity Series: The activity series is very useful and it gives the following informations:
(i) the metal which is higher in the activity series is more reactive than the other. Lithium is the most reactive and platinum is the least reactive metal.
(ii) The metals which have been placed above hydrogen are more reactive than hydrogen and can displace hydrogen from its compounds like water and acids to liberate hydrogen gas.
(iii) The metals which are placed below hydrogen are less reactive than hydrogen and cannot displace hydrogen from its compounds like water and acids.
(iv) A more reactive metal (placed higher in the activity series) can displace the less reactive metal from the solution of its salt.
(v) Metals at the top of the series are very reactive and, therefore, they do not occur free in nature, while the metals at the bottom of the series are least reactive and, therefore, they normally occur free in nature.
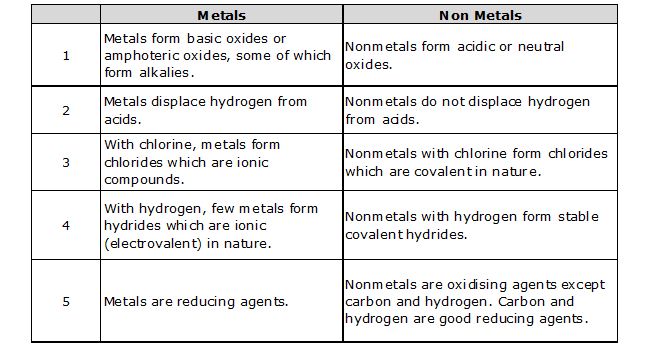
COMPARISON BETWEEN METALS AND NONMETALS
Ex.1 What is galvanisation ? Why is galvanised iron considered better than tin plated iron ? Name the ore of iron which is used to make ships and bridges and give its composition.
Sol. Galvanisation is the process of covering clean iron sheets with zinc by dipping them in molten zinc. Galvanised iron does not rust even if there is a scratch on the zinc layer but if the tin layer gets scratched, the iron starts rusting. Steel is used to make ships and bridges. Composition of steel –(Iron + 0.5 – 1.5 per cent carbon).
Ex.2 List different uses of metal in everyday life.
Sol. Metals are used for making
(i) machinery
(ii) automobiles, aeroplanes, trains, etc.
(iii) pins, cooking utensils, electrical gadgets.
(iv) electrical wires.
(v) thin sheets used for wrapping of food items, medicines, etc.
Ex.3 How will you show that rusting iron needs both the moisture and oxygen
Sol. Take three tubes with fresh iron nail in each. Place a small amount of anhydrous calcium chloride in the first test tube to dry the air. Fill the second test tube completely with boiled water from which dissolved oxygen has been completely removed. Add a small amount of water in the third test tube. Close the mouths of all the three test tubes using rubber stoppers. Keep them undisturbed for 3-4 days.
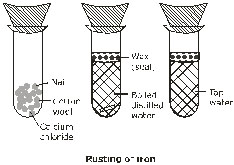
Observe the nails in the test-tubes. You will observe that the nails in the first and second test - tubes are free from rust while the nail in the third test tube has rusted. The above experiment shows that the presence of both air (oxygen) and water is essential for rusting.
Ex.4 Choose appropriate words from the bracket and complete the statements.
(a) Noble gases are found in (free state /compound forms)
(b) Non-metals are generally (malleable /brittle).
(c) Potassium after combustion will form (acidic oxide/ basic oxide).
(d) (Iodine / bromine) has antiseptic properties.
(e) German silver has (copper /silver) as major constituent
Sol. (a) Free state (b) Brittle (c) Basic oxide (d) Iodine (e) Copper
Ex.5 State whether the following statements are true or false :
(a) Sodium is more reactive than magnesium
(b) Magnesium reacts with cold water
(c) All metals exist in solid form at room temperature
(d) Gallium has a low melting point
(e) Gold is alloyed with copper to make it hard
Sol. (a) False (b) True (c) True (d) False (e) True
Ex.6 A set metals in order of their increasing chemical reactivity is given below : silver, copper, lead, iron ,zinc, magnesium and sodium.
(a) Which of the above metals is stored in kerosene ?
(b) Which metals will react with cold water ?
(c) Which gas will be liberated when metals react with cold water ?
(d) Which of the metals will react with oxgyen when heated ?
(e) Which of the metals become black in the presence of hydrogen sulphide ?
Sol. (a) Sodium (b) Sodium (c) Hydrogen (d) Zinc, magneiusm (e) silver
Ex.5 Some properties are listed in the following table. Distinguish between metals and non-metals on the basis of these properties.
Sol.
Ex.6 Give reasons for the following :
(i) Aluminium foils are used to wrap food items
(ii) Immersion rods for heating liquids are made up of metallic substances.
(iii) Copper cannot displace zinc from its salt solution
(iv) Sodium and potassium are stored in kerosene.
Sol. (i) Aluminium is highly malleable metal and it is very easy to make aluminium foil in compare to other metals.
(ii) Immersion rods are made up of metallic substances because metals are good conductors of heat and electricity.
(iii) Copper cannot displace zinc from its solution because zinc is more reactive than copper (i.e., copper is less reactive than zinc.)
(iv) Sodium and potassium metals are very reactive because they react with oxygen and water easily. A lot of heat is produced in the reaction so sodium and potassium are always stored in kerosene.
Ex.7 Can you store lemon pickle in an aluminium utensil? Explain.
Sol. No. this is because acids react with aluminium.
Ex.8 Match the substances given in Column-A with their uses given in Column-B.
Sol.
Ex.9 What happens when
(i) Dilute sulphuric acid is poured on a copper plate?
(ii) Iron nails are placed in copper sulphate solution?
Write word equations of the reactions involved.
Sol. (i) Copper sulphate is formed and hydrogen gas is liberated.
Copper + Sulphuric acid ¾® Copper sulphate + Hydrogen (gas)
(ii) Brown coating is deposited on the iron nails. This is because of the displacement of copper from copper sulphate solution by iron.
Iron + Copper Sulphate (solution) ¾® Iron sulphate (solution) + Copper
Ex.10 Saloni took a piece of burning charcoal and collected the gas evolved in a test tube.
(i) How will she find the nature of the gas?
(ii) Write down word equations of all the reactions taking place in this process.
Sol. (i) She will bring a wet litmus paper in contact with the gas. If the gas turns wet blue litmus paper into red, the gas will be acidic.
(ii) (a) Carbon + Oxygen ¾® Carbon dioxide.
(b) Carbon dioxide + Water ¾® Carbonic acid. (from wet litmus)
Ex.11 One day Reeta went to a jeweller's shop with her mother. Her mother gave an old gold jewellery to the goldsmith to polish. Next day when theybrought the jewellery back, they found that there was slight loss in its weight. Can you suggest a reason for the loss in weight?
Sol. The jeweller dip the jewellery in the solution of acid, which reacts with the outer covering of metals. Thus there is a net loss of weight in the metal of the ornament.
Q.1 Give one example of each: metals and non-metals.
Q.2 Name the metal, which is the best conductor of heat and electricity.
Q.3 Name the property by which metals can be drawn into thin wires.
Q.4 Name the gas produced, when metals react with acids.
Q.5 What is the colour of the copper sulphate solution ?
Q.6 State the nature of oxides of non-metals.
Q.7 Which metal is stored in kerosene ?
Q.8 Name the property of the metal by which it can be drawn into thin sheets.
Q.9 What happens when sulphur reacts with oxygen ?
Q.10 Which non-metal catches fire, if exposed to air ?
Q.11 Name the gas that burns with a POP sound.
Q.12 What are Displacement reactions ?
Q.13 Give one use of non-metal in our daily life.
Q.14 What are metalloids ?
Q.15 Which metal is use to wrap food items
Q.16 What happens when sulphur di-oxide reacts with water ? Give the chemical reaction involved.
Q.17 Why lemon pickle cannot be stored in an aluminium foil ?
Q.18 Write two important properties of metals.
Q.19 Why copper cannot displace zinc from zinc sulphate solution ?
Q.20 Why immersion rods for heating are made up of metallic substances ?
Q.21 What happens when iron nails are dipped in water in a test tube for a week ?
Q.22 What happens when iron reacts with oxygen and water ? Give the chemical reaction involved.
Q.23 What happens when copper vessel is exposed to moist air for a long time ? Give the chemical reaction that takes place.
Q.24 Why gold is preferred in making jewellery
Q.25 What happens when dilute sulphuric acid is poured on a zinc plate ? Write the chemical
reaction takes place.
Q.26 What happens when magnesium ribbon is burnt in air ?
Q.27 Why metals are used in making aeroplanes, bridges, satellites etc.
Q.28 Complete the following chemical reactions.
(i) Zn + H2SO4 ®
(iv) 2Cu + H2O + CO2
(ii) 2Fe + O2 + H2O ®
(v) SO2 + H2O ®
(iii) Cu + HCl ®
Q.29 what will happen when ash of magnesium is dissolved in water ? Is the solution acidic or basic ? What effect does litmus show in case of oxides of metals ?
Q.30 Explain the following terms :
(i) Malleability (ii) Ductility (iii) Sonorous
(iv) Lustrous (v) Metalloids.
Q.31 Gold dissolves in (aqua regia / aqueous solution of silver nitrate).
Q.32 Silver tarnishes due to (nitrogen oxides /hydrogen sulphide) in the air.
Q.33 Name the components of stainless steel.
Q.34 what are the constituents of bronze ?
Q.35 Define the term alloy.
Q.36 What are the components of solder ?
Q.37 What do you understand by the term corrosion ?
Q.38 Describe the process of galvanization.
Q.39 What is the action of water on :
(i) Magnesium (ii) Sodium (iii) Iron
Q.40 What type of oxides are formed by non - metals ?
Q.41 What is the advantage of using stainless steel for making utensils as compared to iron
Q.42 Give the composition of brass.
Q.43 Define rusting.
Q.44 How can bridges be protected from rusting
Q.45 How are machines protected from rusting ?
Q.46 Name the alloy of aluminium and magnesium.
Q.47 What name is given to the alloy fo alumminium copper, manganese and magnesium ?
Q.48 Name the soft metal which can be cut with a knife.
Q.49 Name the non-metal used in vulcanization.
Q.50 Name one non-metal which has lustre.
Q.51 Name one non-metal which is good conductor of electricity.
Q.1 Who classified the elements in metals and non-metals?
(A) Lavoisier (B) Priestley
(C) Lemaitre (D) Lenoir
Q.2 Which of the following metals occur in their pure state?
(A) Copper (B) Iron
(C) Zinc (D) Gold
Q.3 Which of the following metals is liquid at room temperature?
(A) Sodium (B) Mercury
(C) Zinc (D) Aluminium
Q.4 Which of the following is a good conductor of heat?
(A) Bromine (B) Chlorine
(C) Mercury (D) Iodine
Q.5 Which of the following non-metals occurs as liquid?
(A) Bromine (B) Sulphur
(C) Iodine (D) Carbon
Q.6 Which of the following non-metals occurs as a solid?
(A) Sulphur (B) Carbon
(C) Iodine (D) All of the above
Q.7 Which of the following non-metal occurs as a gas?
(A) Nitrogen (B) Chlorine
(C) Both the above (D) None of the above
Q.8 Which of the following metals has very low melting point and melts even in hand?
(A) Sodium (B) Gallium
(C) Potassium (D) Graphite
Q.9 Which of the following is lighter than water?
(A) Potassium (B) Sulphur
(C) Iodine (D) Graphite
Q.10 Which of the following is denser than many metals?
(A) Bromine (B) Chlorine
(C) Sulphur (D) Iodine
Q.11 Which non-metal is a good conductor of electricity?
(A) Bromine (B) Iodine
(C) Graphite (D) Chlorine
Q.12 Magnesium oxide is –
(A) Basic oxide (B) Acidic oxide (C) Neutral oxide (D) None of these
Q.13 Sulphur dioxide is –
(A) Basic oxide (B) Acidic oxide (C) Neutral oxide (D) None of these
Q.14 Which of the following is a noble metal?
(A) Copper (B) Iron
(C) Gold (D) Aluminium
Q.15 When medium reacts with cold water, it forms–
(A) Sodium hydroxide and oxygen
(B) Sodium hydroxide and hydrogen
(C) Sodium hydroxide and carbon dioxide
(D) None of these
Q.16 Which of the following is very reactive and kept in kerosene?
(A) Iodine (B) Bromine
(C) Sodium (D) Potassium
Q.17 Which of the following reacts violently with steam?
(A) Iron (B) Zinc
(C) Magnesium (D) None of these
Q.18 Which metal reacts with dilute hydrochloric acid to produce hydrogen?
(A) Zinc (B) Copper
(C) Iron (D) Platinum
Q.19 When zinc is put in copper sulphate solution, the colour of copper sulphate becomes –
(A) Greenish (B) Yellowish
(C) Brownish (D) Colourless
Q.20 When iron nail is placed in copper sulphate solution, the bluish colour of copper sulphate turns –
(A) Brownish (B) Yellowish
(C) Greenish (D) Colourless
1. A 2. D 3. B 4. C 5. A
6. D 7. C 8. B 9. A 10. D
11. C 12. A 13. B 14. C 15. B
16. C 17. C 18. A 19. D 20. C
1. Rusting of iron can be prevented by :-
(A) Alloying (B) Painting (C) Galvaning (D) All of these
2. Which of the following is a good conductors of heat and electricity ?
(A) Graphite (B) Oxygen (C) Chlorine (D) Nitrogen
3. Metals are :-
(A) Malleable (B) Ductile (C) None (D) Both
4. Metals can be obtained economically from:-
(A) Minerals (B) Ores (C) Earth's crust (D) None
5. Which of the following have low melting and boiling points ?
(A) Phosphorus (B) Sodium (C) Iron (D) (A) and (B)
6. Which of the following metals catch fire on reaction with water ?
(A) Sodium (B) Potassium (C) Magnesium (D) (A) and (B)
7. A metal, which forms a protective layer of its oxide on reaction with water, on its surface is :-
(A) Sodium (B) Aluminium (C) Potassium (D) Magnesium
8. Reactivity series gives :-
(A) Arrangement of metals in the order of decreasing reactivity
(B) Arrangement of non-metals in the order of decreasing reactivity
(C) Arrangement of metals in the order of increasing reactivity
(D) Arrangement of non-metals in the order of increasing reactivity
9. Metals like gold, platinum which do not easily react are called :-
(A) Active metals (B) Dull metals (C) Noble metals (D) Bright metals
10. When MgO is dissolved in water, Mg(OH)2 is obtained. A red litmus paper dipped in this solution turns blue, this shows that the solution is ............ in nature :-
(A) Acidic (B) Neutral (C) Alkaline (D) Reactive
11. Calgon is a substance which is used to remove the hardness of water. The formula of calgon is :-
(A) Na2[Na4(PO3)6] (B) Na2Al2Si2O8
(C) FeSO4(NH4)2 . SO4 . 6H2O (D) Na2S2O3 . 5H2O
12. Type metal is an alloy of :-
(A) Pb and Sn (B) Pb, Sn and Sb (C) Cu and Zn (D) Cu, Sn and Sb
13. Lightest liquid metal is :-
(A) Hg (B) Cs (C) Ga (D) None of these
14. 90% of sun's mass is :-
(A) He (B) H2 (C) O2 (D) Ar
15. Carnallite (KCl . MgCl2 . 6H2O) is an ore of :-
(A) Chlorine (B) Iodine (C) Astatine (D) Bromine
16. Magnetite is an oxide of metal X. The metal is :-
(A) Mn (B) Mg (C) Fe (D) Cu
17. Hoope's process is used for the purification of
(A) Cu (B) Fe (C) Al (D) At
18. Mercury is also called :-
(A) Liquid gold (B) Liquid copper (C) Liquid silver (D) None of these
19. Nitric acid can be prepared by which of the following method ?
(A) Birkeland - eyde process (B) Ostwald process
(C) Contact process (D) (A) & (B) both
20. Which of the following is/are used as bleaching agent ?
(A) SO2 (B) H2S (C) Cl2 (D) All
21. Which of the following element is not found in free state in the nature ?
(A) Silver (B) Copper (C) Sodium (D) Gold
22. When magma cools below the surface of the earth, the granite is formed which is used in buildings. It mainly consists of :-
(A) Quartz and haematite (B) Quartz and felspar
(C) Bauxite and calcamine (D) Felspar and silver glance
23. Minerals generally have the following characteristics :-
(a) They occur naturally
(b) They have characteristics chemical composition
(c) They do not have specific chemical properties
(d) They do not have a specific chemical composition
(A) (a) and (b) (B) (a) and (c) (C) (c) and (d) (D) (a) and (d)
24. The percentage of gold present in 20 carat gold is :-
(A) 83.33 (B) 100 (C) 50 (D) 73.3
25. Which of the following elements is non-metal ?
(A) Na (B) Fe (C) Cu (D) S
26. When a compound A is heated, a gas B is evolved which turns lime water milky. Compound A is used in the manufacture of glass. Gas B has a property of extinguishing fire and it does not support animal life. The compound A and B are respectively :-
(A) NaHCO3 and CO (B) CaCO3 and CO (C) Na2CO3 and CO2 (D) NaHCO3 and CO2
27. Which of the following non-metal is found in liquid state at room temperature ?
(A) Sulphur (B) Carbon (C) Iodine (D) Bromine
28. Match the following :-
(a) CH4 (i) Neither combustible nor supports combustion
(b) CO2 (ii) Combustible
(c) N2 (iii) Supports combustion
(d) O2 (iv) Extinguishes fire
Which of the following indicates the correct matching ?
(A) (a)-(i) ; (b)-(ii) ; (c)-(iii) ; d-(iv) (B) (a)-(ii) ; (b)-(iv) ; (c)-(i) ; d-(iii)
(C) (a)-(ii) ; (b)-(iii) ; (c)-(i) ; d-(iv) (D) (a)-(iii) ; (b)-(iv) ; (c)-(ii) ; d-(i)
29. Which of the following displacement reactions is possible ?
(A) Copper + Sodium chloride ¾® Copper chloride + Sodium
(B) Lead + Potassium nitrate ¾® Lead nitrate + Potassium
(C) Iron + Lead nitrate ¾® Iron nitrate + Lead
(D) Silver + Copper nitrate ¾® Silver nitrate + Copper
30. Essential component of an amalgam is -
(A) An alkali metal (B) Mercury (C) Silver (D) A non-metal
31. The red or Orange coating that forms on the surface or iron when exposed to air and moisture for some time is called-
(A) Galvanisation (B) Electroplating (C) Rust (D) Reduction
32. The tip of the lead pencil is made of -
(A) Lead (B) Graphite (C) Zinc (D) Charcoal
33. The most abundant element in the sun's atmosphere is -
(A) Xenon (B) Argon (C) Hydrogen (D) Oxygen
34. White phosphorus is stored in -
(A) Ether (B) Water (C) Alcohol (D) Kerosene Oil
35. Hydrogen gas is passed through oil in order to
(A) Convert lower oil to higher oil
(B) Convert liquid oil into solidified oil
(C) Convert unsaturated hydrocarbon to saturated hydrocarbon
(D) All the above statements are wrong
36. Metals are-
(A) Element which can lose electron easily forming positive ions
(B) Element which can gain electron easily forming negative ions
(C) Elements
(D) None of these
37. The element containing 1, 2 or 3 valence electrons are -
(A) Metal (B) Non-metal (C) Noble element (D) None of these
38. The element which shows the characteristics of both metal and non-metal are -
(A) Electropositive elements (B) Electronegative elements
(C) Metalloids (D) Galvanization
39. Extraction of metals from their respective ores is called -
(A) Metallurgy (B) Galvanization (C) Malleability (D) Galvanization
40. The earthy impurities present along with the mineral in an ore is called -
(A) Alloy (B) Waste material (C) Waste materials (D) Gangue or matrix
41. Cinnabar is an ore of -
(A) Pb (B) Zn (C) Hg (D) Cu
42. A metal obtained by auto reduction of self reduction -
(A) Na (B) Zn (C) Fe (D) Cu
43. Which of these is obtained by electrolytic reduction of its compound-
(A) Cu (B) Gold (C) Mg (D) Bi
44. Which of them is not an ore of silver -
(A) Ag2S (B) AgNO3 (C) AgCl (D) None of them
45. The metal refined-electrolytically is -
(A) Al (B) Na (C) Cu (D) Fe
46. The reaction used to join railway tracks involves reducing agent-
(A) Al (B) Mg (C) C (D) CO
47. In the blast furnace/ the flux is -
(A) Acidic (B) Basic (C) Amphoteric (D) Not matter
48. Liquation is used to refine -
(A) Iron (B) Tin (C) Copper (D) Gold
49. In the metallurgy of iron using blast furnace the slag is-
(A) FeSiO3 (B) CaSiO3 (C) CaCO3 (D) CaSO3
50. Zinc Blende is concentrated by-
(A) Chemical separation (B) Magnetic sepciation (C) Froth floatation (D) Hydraulic washing
51. Which of the following alloyes contains Tin -
(A) Brass (B) Solder (C) Duralumin (D) Steel
52. The iron obtained from blast furnace is -
(A) Steel (B) Cast iron (C) Pig iron (D) Wrought iron
53. An amalgam of metal has which other element-
(A) C (B) Ag (C) Mg (D) Hg
54. Gold as alloyed with which metal to make it harder-
(A) Cu (B) Hg (C) Ag (D) C
55. Which of these metals canot be obtained by reduction using C as reducing agent -
(A) Copper (B) Zinc (C) Lead (D) Potassium
56. The most abundant element in the universe is
(A) Helium (B) Oxygen (C) Silicon (D) Hydrogen
57. The most abundant metal on the earth is-
(A) Iron (B) Gold (C) Aluminium (D) Copper
58. Aluminium is extracted from -
(A) Hematite (B) Bauxite (C) Calamine (D) Magnetite
59. Metallurgy is the process of-
(A) Extracting metals from the ore
(B) Roasting the ore
(C) Liquefaction of nitrogen (D) Adding carbon to the ore in blast furnace
60. A metal, whcih exists in the liquid state-
(A) Potassium (B) Sodium (C) Mercury (D) Gallium
61. A metal, which melts on the palm-
(A) Potassium (B) Sodium (C) Gallium (D) Zinc
62. The process of protecting iron by coating with zinc-
(A) Smelting (B) Galvanising (C) Rusting (D) Corrosion
63. The metalloids include the elements -
(A) Boron, Silicon (B) Arsenic, Antimony (C) Germanium, Tellurium (D) All the above
64. Metals generally form -
(A) basic oxides (B) acidic oxides (C) neutral (D) none
65. Which element is an important component of transistors-
(A) Sodium (B) Copper (C) Germanium (D) Radium
66. A lustrous non-metal is -
(A) Diamond (B) Iodine (C) Sulphur (D) Phosphorus
67. Metals are -
(A) Bad conductors of heat and electricity
(B) Good conductors of heat and electricity
(C) Good conductors of heat and bad conductors of electricity
(D) Semi conductor of heat and electricity
68. Metals are-
(A) Malleable and ductile
(B) Non-malleable and ductile
(C) Brittle and ductile (D) Non-malleable and non-ductile
69. Metals have-
(A) High melting and boiling point
(B) Low melting and boiling point
(C) High melting and low boiling point (D) Low melting and high boiling point
70. Arrange the following metal in the increasing order of their reactivity towards water Zinc, Iron, Magnesium, Sodium-
(A) Iron < Magnesium < Sodium < Zinc
(B) Iron < Zinc < Magnesium < Sodium
(C) Magnesium < Iron < Sodium < Zinc (D) Sodium < Iron < Magnesium < Zinc
1. D 2. A 3. D 4. B
5. D 6. D 7. B 8. A
9. C 10. A 11. A 12. D
13. B D14. B 15. D 16. C
17. C 18. C 19. D 20. D
21. C 22. B 23. B 24. A
25. D 26. D 27. D 28. B
29. C 30. B 31. C 32. B
33. C 34. B 35. C 36. A
37. A 38. C 39. A 40. D
41. C 42. D 43. C 44. D
45. C 46. A 47. B 48. B
49. B 50. C 51. B 52. C
53. D 54. A 55. D 56. B
57. C 58. B 59. A 60. C
61. B 62. B 63. A 64. A
65. C 66. B 67. B 68. A
69. A 70. B