Exercise
1. Calculate the volume of 1 mole of an ideal gas at STP.
Ans. 2.24 × 10–2 m3
2. Find the number of molecules of an ideal gas in a volume of 1.000 cm3 at STP.
Ans. 2.685 × 1019
3. Find the number of molecules in 1 cm3 of an ideal gas at 0ºC and at a pressure of 10–5 mm of mercury.
Ans. 3.53 × 1011
4. Calculate the mass of 1 cm 3 of oxygen kept at STP.
Ans. 1.43 mg
5. Equal masses of air are sealed in two vessels, one of volume V0 and the other of volume 2V0. If the first vessel is
maintained at a temperature 300 K and the other at 600 K, find the ratio of the pressures in the two vessels.
Ans. 1 : 1
6. An electric bulb of volume 250 cc was sealed during manufacturing at a pressure of 10–3 mm of mercury at 27ºC.
Complete the number of air molecules contained in the bulb. Avogadro constant = 6 × 10 23 per mol, density of mercury
= 136000 kg/m3 and g = 10 m/s2.
Ans. 8.0 × 1015
7. A gas cylinder has walls that can bear a maximum pressure of 1.0 × 10 6 Pa. It contains a gas at 8.0 × 10 5 Pa and 300 K.
The cylinder is steadily heated. Neglecting any change in the volume, calculate the temperature at which the cylinder will break.
Ans. 375 K
8. 2 g of hydrogen is sealed in a vessel of volume 0.02 m 3 and is maintained at 300 K. Calculate the pressure in the vessel.
Ans. 1.24 × 105 Pa
9. The density of an ideal gas is 1.25 × 10–3 g/cm3 at STP. Calculate the molecular weight of the gas.
Ans. 28.3 g/mol
10. The temperature and pressure at Simla are 15.0ºC and 72.0 cm of mercury and at Kalka these are 35ºC and 76.0 cm of mercury.
Find the ratio of air density at Kalka to the air density at Simla.
Ans. 0.987
11. Figure shows a cylindrical tube with adiabatic walls and fitted with a diathermic separator. The separator can be slid in the tube
by an external mechanism. An ideal gas is injected in the two sides at equal pressures and equal temperatures. The separator remains
in equilibrium at the middle. It is now slid to a position where it divides the tube in the ratio of 1 : 3. Find the ratio of the pressures in
the two parts of the vessel.

Ans. 3 : 1
12. Find the rms speed of hydrogen molecules in a sample of hydrogen gas at 300 K. Find the temperature at which the rms speed is
double the speed calculated in the previous part.
Ans. 1930 m/s, 1200 K
13. A sample of 0.177 g of an ideal gas occupies 1000 cm3 at STP. Calculate the rms speed of the gas molecules.
Ans. 1300 m/s
14. The average translational kinetic energy of air molecules is 0.040 eV (1 eV = 1.6 × 10–19 J). Calculate the temperature of the air.
Boltzmann constant k = 1.38 × 10–23 J/K.
Ans. 310 K
15. Consider a sample of oxygen at 300 K. Find the average time taken by a molecule to travel a distance equal to the diameter of the earth.
Ans. 8.0 hour
16. Find the average magnitude of linear momentum of a helium molecule in a sample of helium gas at 0ºC. Mass of a helium molecule
= 6.64 × 10–27 kg and Boltzmann constant = 1.38 × 10 –23 J/K.
Ans. 8.0 × 10–24 kg–m/s
17. The mean speed of the molecules of a hydrogen sample equals the mean speed of the molecules of a helium sample. Calculate the ratio
of the temperature of the hydrogen sample to the temperature of the helium sample.
Ans. 1 : 2
18. At what temperature the mean speed of the molecules of hydrogen gas equals the escape speed from the earth?
Ans. 11800 K
19. Find the ratio of the mean speed of hydrogen molecules to the mean speed of nitrogen molecules in a sample containing a mixture of the two gases.
Ans. 3.74
20. Figure shows a vessel partitioned by a fixed diathermic separator. Different ideal gases are filled in the two parts. The rms speed of the molecules
in the left part equals the mean speed of the molecules in the right part. Calculate the ratio of the mass of a molecule in the left part of the mass
of molecule in the right part.

Ans. 1.18
21. Estimate the number of collisions per second suffered by a molecules in a sample of hydrogen at STP. The mean free path
(average distance covered by a molecule between successive collisions) = 1.38 × 10–5 cm.
Ans. 1.23 × 10 10
22. Hydrogen gas is contained in a closed vessel at 1 atm (100 kPa) and 300 K. (a) Calculate the mean speed of the molecules.
(b) Suppose the molecules strike the wall with this speed making an average angle of 45º with it. How many molecules strike
each square metre of the wall per second?
Ans. (a) 1780 m/s (b) 1.2 × 10 28
23. Air is pumped into an automobile tyre’s tube upto a pressure of 200 kPa in the morning when the air temperature is 20ºC.
During the day the temperature rises to 40ºC and the tube expands by 2%. Calculate the pressure of the air in the tube at this temperature.
Ans. 209 kPa
24. Oxygen is filled in a closed metal jar of volume 1.0 × 10–3 m–3 at a pressure of 1.5 × 10 5 Pa and temperature 400 K. The jar has a small leak in it.
The atmospheric pressure is 1.0 × 105 Pa and the atmospheric temperature is 300 K. Find the mass of the gas that leaks out by the time the pressure
and the temperature inside the jar equalise with the surrounding.
Ans. 0.16 g
25. An air bubble of radius 2.0 mm is formed at the bottom of a 3.3 m deep river. Calculate the radius of the bubble as it comes to the surface.
Atmospheric pressure = 1.0 × 10 5 Pa and density of water = 1000 kg/m3.
Ans. 2.2 mm
26. Air is pumped into the tubes of a cycle rickshaw at a pressure of 2 atm. The volume of each tube at this pressure is 0.002 m3.
One of the tubes gets punctured and the volume of the tube reduces to 0.0005 m3. How many moles of air have leaked out?
Assume that the temperature remains constant at 300 K and that the air behaves as an ideal gas.
Ans. 0.14
27. 0.040 g of He is kept in a closed container initially at 100.0ºC. The container is now heated. Neglecting the expansion of the container,
calculate the temperature at which the internal energy is increased by 12 J.
Ans. 196ºC
28. During an experiment, an ideal gas is found to obey an additional law pV2 = constant. The gas is initially at a temperature T and volume V.
Find the temperature when it expands to a volume 2V.
Ans. T/2
29. A vessel contains 1.60 g of oxygen and 2.80 g of nitrogen. The temperature is maintained at 300 K and the volume of the vessel is 0.166 m3.
Find the pressure of the mixture.
Ans. 2250 N/m2
30. A vertical cylinder of height 100 cm contains air at a constant temperature. The top is closed by a frictionless light piston.
The atmospheric pressure is equal to 75 cm of mercury. Mercury is slowly poured over the piston. Find the maximum height
of the mercury column that can be put on the piston.
Ans. 25 cm
31. Figure shows two vessels A and B with rigid walls containing ideal gases. The pressure, temperature and the volume are pA, TA,
V in the vessel A and pB, TB, V in the vessel B. The vessels are now connected through a small tube. Show that the pressure p
and the temperature T satisfy
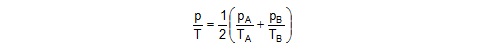
when equilibrium is achieved.
32. A container of volume 50 cc contains air (mean molecular weight = 28.8 g) and is open to atmosphere where the pressure is
100 kPa. The container is kept in a bath containing melting ice (0ºC).
(a) Find the mass of the air in the container when thermal equilibrium is reached.
(b) The container is now placed in another bath containing boiling water (100ºC). Find the mass of air in the container.
(c) The container is now closed and placed in the melting-ice bath. Find the pressure of the air when thermal equilibrium is reached.
Ans. (a) 0.058 g (b) 0.0468 g (c) 73.0 kPa
33. A uniform tube closed at one end, contains a pallet of mercury 10 cm long. When the tube is kept vertically with the closed end upward,
the length of the air column trapped is 20 cm. Find the length of the air column trapped when the tube is inverted so that the closed end
goes down. Atmospheric pressure = 75 cm of mercury.
Ans. 15 cm
34. A glass tube, sealed at both ends, is 100 cm long. It lies horizontally with the middle 10 cm containing mercury. The two ends of the tube contain air
at 27ºC and at a pressure 76 cm of mercury. The air column on one side is maintained at 0ºC and the other side is maintained at 127ºC.
Calculate the length of the air column on the cooler side. Neglect the changes in the volume of mercury and of the glass.
Ans. 36.5 cm
35. An ideal gas is trapped between a mercury column and the closed end of a narrow vertical tube of uniform base containing the column.
The upper end of the tube is open to the atmosphere. The lengths of the mercury column and the trapped air column are 20 cm and 43 cm respectively.
What will be the length of the air column when the tube is tilted slowly in a vertical plane through an angle of 60º?
Assume the temperature to remain constant.
Ans. 48 cm
36. Figure shows a cylindrical tube of length 30 cm which is partitioned by a tight-fitting separator. The separator is very weakly conducting and
can freely slide along the tube. Ideal gases are filled in the two parts of the vessel. In the beginning the temperatures in the parts A and B are
400 K and 100 K respectively. The separator slides to a momentary equilibrium position shown in the figure. Find the final equilibrium position
of the separator, reached after a long times.

Ans. 10 cm from the left end
37. A vessel of volume V0 contains an ideal gas at pressure p0 and temperature T. Gas is continuously pumped out of this vessel at a constant
volume – rate dV/dt = r keeping the temperature constant. The pressure of the gas being taken out equals the pressure inside the vessel. Find
(a) the pressure of the gas as a function of time,
(b) the time taken before half the original gas is pumped out.
Ans. (a) p = p0
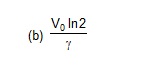

39. Show that the internal energy of the air (treated as an ideal gas) contained in a room remains constant as the temperature changes
between day and night. Assume that the atmospheric pressure around remains constant and the air in the room maintains this pressure
by communicating with the surrounding through the windows etc.
40. Figure shows a cylindrical tube of radius 5 cm and length 20 cm. It is closed by a tight-fitting cork. The friction coefficient between the cork and the tube is 0.20. The tube contains an ideal gas at a pressure of 1 atm and a temperature of 300 K. The tube is slowly heated and it is found that the cork pops out when the temperature reaches 600 K. Let dN denote the magnitude of the normal contact force exerted by a small length dl, of the cork along the periphery (see the figure). Assuming that the temperature of the gas is uniform at any instant, calculate dN/dl.
Ans. 1.25 × 10 4 N/m
41. Figure shows a cylindrical tube of cross-sectional area A fitted with two frictionless pistons. The pistons are connected to each other by a metallic wire. Initially, the temperature of the gas is T0 and its pressure is p0 which equals the atmospheric pressure. (a) What is the tension in the wire? (b) What will be the tension if the temperature is increased to 2T0 ?
Ans. (a) zero (b) p0 A
42. Figure shows a large closed cylindrical tank containing water. Initially the air trapped above the water surface has a height h0 and pressure 2p0 where p0 is the atmospheric pressure. There is a hole in the wall of the tank at a depth h1 below the top from which water comes out. A long vertical tube is connected as shown. (a) Find the height h2 of the water in the long tube above the top initially. (b) Find the speed with which water comes out of the hole. (c) Find the height of the water in the long tube above the top when the water stops coming out of the hole.
43. An ideal gas is kept in a long cylindrical vessel fitted with a frictionless piston of cross-sectional area 10 cm2 and weight 1 kg (figure). The vessel itself is kept in a big chamber containing air at atmospheric pressure 100 kPa. The length of the gas column is 20 cm. If the chamber is now completely evacuated by an exhaust pump, what will be the length of the gas column? Assume the temperature to remain constant throughout the process.
Ans. 2.2 m
44. An ideal gas is kept in a long cylindrical vessel fitted with a frictionless piston of cross-sectional area 10 cm2 and weight 1 kg. The length of the gas column in the vessel is 20 cm. The atmospheric pressure is 100 kPa. The vessel is now taken into a spaceship revolving round the earth as a satellite. The air pressure in the spaceship is maintained at 100 kPa. Find the length of the gas column in the cylinder.
Ans. 22 cm
45. Two glass bulbs of equal volume are connected by a narrow tube and are filled with a gas at 0ºC at a pressure of 76 cm of mercury. One of the bulbs is then placed in melting ice and the other is placed in a water bath maintained at 62ºC. What is the new value of the pressure inside the bulbs? The volume of the connecting tube is negligible.
Ans. 84 cm of mercury
46. The weather report reads, “Temperature 20ºC : Relative humidity 100%”. What is the dew point?
Ans. 20ºC
47. The condition of air in a closed room is described as follows. Temperature = 25ºC, relative humidity = 60%, pressure = 104 kPa. If all the water vapour is removed from the room without changing the temperature, what will be the new pressure? The saturation vapour pressure at 25ºC = 3.2 kPa.
Ans. 102 kPa
48. The temperature and the dew point in an open room are 20ºC and 10ºC. If the room temperature drops to 15ºC, what will be the new dew point?
Ans. 10ºC
49. Pure water vapour is trapped in a vessel of volume 10 cm3. The relative humidity is 40%. The vapour is compressed slowly and isothermally. Find the volume of the vapour at which it will start condensing.
Ans. 4.0 cm3
50. A barometer tube is 80 cm long (above the mercury reservoir). It reads 76 cm on a particular day. A small amount of water is introduced in the tube and the reading drops to 75.4 cm. Find the relative humidity in the space above the mercury column if the saturation vapour pressure at the room temperature is 1.0 cm.
Ans. 60%
51. Using figure, find the boiling point of methyl alcohol at 1 atm (760 mm of mercury) and at 0.5 atm.
Ans. 65ºC, 48ºC
52. The human body has an average temperature of 98º F. Assume that the vapour pressure of the blood in the veins behaves like that of pure water. Find the minimum atmospheric pressure which is necessary to prevent the blood from boiling. Use figure for the vapour pressures.
Ans. 50 mm of mercury
53. A glass contains some water at room temperature 20ºC. Refrigerated water is added to it slowly. When the temperature of the glass reaches 10ºC, small droplets condense on the outer surface. Calculate the relative humidity in the room. The boiling point of water at a pressure of 17.5 mm of mercury is 20ºC and at 8.9 mm of mercury it is 10ºC.
Ans. 51%
54. 50 m3 of saturated vapour is cooled down from 30ºC to 20ºC. Find the mass of the water condensed. The absolute humidity of saturated water vapour is 30 g/m3 at 30ºC and 16 g/m3 at 20ºC.
Ans. 700 g
55. A barometer correctly reads the atmospheric pressure as 76 cm of mercury. Water droplets are slowly introduced into the barometer tube by a dropper. The height of the mercury column first decreases and then becomes constant. If the saturation vapour pressure at the atmospheric temperature is 0.80 cm of mercury, find the height of the mercury column when it reaches its minimum value.
Ans. 75.2 cm
56. 50 cc of oxygen is collected in an inverted gas jar over water. The atmospheric pressure is 99.4 kPa and the room temperature is 27ºC. The water level in the jar is same as the level outside. The saturation vapour pressure at 27ºC is 3.4 kPa. Calculate the number of moles of oxygen collected in the jar.
Ans. 1.93 × 10–3
57. A faulty barometer contains certain amount of air and saturated water vapour. It reads 74.0 cm when the atmospheric pressure is 76.0 cm of mercury and reads 72.10 cm when the atmospheric pressure is 74.0 cm of mercury. Saturation vapour pressure at the air temperature = 1.0 cm of mercury. Find the length of the barometer tube above the mercury level in the reservoir.
Ans. 91.1 cm
58. On a winter day, the outside temperature is 0ºC and relative humidity 40%. The air from outside comes into a room and is heated to 20ºC. What is the relative humidity in the room? The saturation vapour pressure at 0ºC is 4.6 mm of mercury and at 20ºC it is 18 mm of mercury.
Ans. 9.5%
59. The temperature and humidity of air are 27ºC and 50% on a particular day. Calculate the amount of vapour that should be added to 1 cubic metre of air to saturate it. The saturation vapour pressure at 27ºC = 3600 Pa.
Ans. 13 g
60. The temperature and relative humidity in a room are 300 K and 20% respectively. The volume of the room is 50 m3. The saturation vapour pressure at 300 K is 3.3 kPa. Calculate the mass of the water vapour present in the room.
Ans. 238 g
61. The temperature and the relative humidity are 300 K and 20% in a room of volume 50 m3. The floor is washed with water, 500 g of water sticking on the floor. Assuming no communication with the surrounding, find the relative humidity when the floor dries. The changes in temperature and pressure may be neglected. Saturation vapour pressure at 300 K = 3.3 kPa.
Ans. 62%
62. A bucket full of water is placed in a room at 15ºC with initial relative humidity 40%. The volume of the room is 50 m3. (a) How much water will evaporate? (b) If the room temperature is increased by 5ºC how much more water will evaporate? The saturation vapour pressure of water at 15ºC and 20ºC are 1.6 kPa and 2.4 kPa respectively.
Ans. (a) 361 g (b) 296 g
Extra questions
8. Find the number of molecules of an ideal gas in a volume of 1.000 cm3 at STP.
Ans. 2.685 × 1019
9. An ideal gas is kept in a long cylindrical vessel fitted with a frictionless piston of cross-sectional area 10 cm2 and weight 1 kg. The length of the gas column in the vessel is 20 cm. The atmospheric pressure is 100 kPa. The vessel is now taken into a spaceship revolving round the earth as a satellite. The air pressure in the spaceship is maintained at 100 kPa. Find the length of the gas column in the cylinder.
Ans. 22 cm
10. Consider a sample of oxygen at 300 K. Find the average time taken by a molecule to travel a distance equal to the diameter of the earth.
Ans. 8.0 hr.
11. Find the average magnitude of linear momentum of a helium molecule in a sample of helium gas at 0ºC. Mass of a helium molecule = 6.64 × 10–27 kg and Boltazmann constant = 1.38 × 10–23 J/K.
Ans. 8.0 × 10–24 kg-m/s
12. During an experiment, an ideal gas is found to obey an additional law pV2 = constant. The gas is initially at a temperature T and volume V. Find the temperature when it expands to a volume 2V.
Ans. T/ 2
13. Calculate the heat absorbed by a system in going through the cyclic process shown in figure.
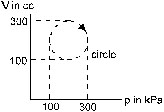
Ans. 31.4 J
14. Consider the cyclic process ABCA, shown in figure, performed on a sample of 2.0 mole of an ideal gas. A total of 1200 J of heat is withdrawn from the sample in the process Find the work done by the gas during the part BC. 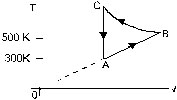
Ans. –4520 J
15. A substance is taken through the process abc as shown in figure. If the internal energy of the substance increases by 5000 J and a heat of 2625 cal is given to the system, calculate the value of J.
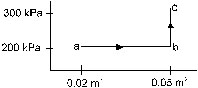
Ans. 4.19 J/cal.
16. A gas is taken along the path AB as shown in figure. If 70 cal of heat is extracted from the gas in the process, calculate the change in the internal energy of the system.
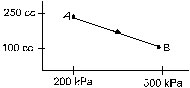
Ans. –241 J
17. Estimate the number of collisions per second suffered by a molecule in a sample of hydrogen at STP. The mean free path (average distance covered by a molecule between successive collisions) = 1.38 × 10–5 cm. Ans. 1.23 × 1010
18. Hydrogen gas is contained in a closed vessel at 1 atm (100 kPa) and 300 K.
(a) Calculate the mean speed of the molecules.
(b) Suppose the molecules strike the wall with this speed making an average angle of 45º with it. How many molecules strike each square metre of the wall per second ?
Ans. (a) 1780 m/s (b) 1.2 × 1028
19. A vertical cylinder of height 100 cm contains air at a constant temperature. The top is closed by a frictionless light piston. The atmospheric pressure is equal to 75 cm of mercury. Mercury is slowly poured over the piston. Find the maximum height of the mercury column that can be put on the piston.
Ans. 25 cm
21. Figure shows a cylindrical tube of volume V0 divided in two parts by a frictionless separator. The walls of the tube are adiabatic but the separator is conducting. Ideal gases are filled in the two parts. When the separator is kept in the middle, the pressures are p1 and p2 in the left part and the right part respectively. The separator is slowly slid and is released at a position where it can stay in equilibrium. Find the volumes of the two parts.
Ans. V2 = p2V0/p1 + p2
22. A vessel of volume V0 contains an ideal gas at pressure p0 and temperature T. Gas is continuously pumped out of this vessel at a constant volume-rate dV/dt = r keeping the temperature constant. The pressure of the gas being taken out equals the pressure inside the vessel. Find
(a) the pressure of the gas as a function of time,
(b) the time taken before half the original gas is pumped out.

24. A horizontal tube of length l closed at both ends contains an ideal gas of molecular weight M. The tube is rotated at a constant angular velocity w about a vertical axis passing through an end. Assuming the temperature to be uniform and constant, show that

25. Figure shows a cylindrical tube of volume V with adiabatic walls containing an ideal gas. The internal energy of this ideal gas is given by 1.5 nRT. The tube is divided into two equal parts by a fixed diathermic wall. Initially, the pressure and the temperature are p1, T1 on the left and p2, T2 on the right. The system is left for sufficient time so that the temperature becomes equal on the two sides. (a) How [5]

26. A 100 kg block is started with a speed of 2.0 m/s on a long, rough belt kept fixed in a horizontal position. The coefficient of kinetic friction between the block and the belt is 0.20.
(a) Calculate the change in the internal energy of the block-belt system as the block comes to a stop on the belt.
(b) Consider the situation from a frame of reference moving at 2.0 m/s along the initial velocity of the block. As seen from this frame, the block is gently put on a moving belt and in due time the block starts moving with the belt at 2.0 m/s. Calculate the increase in the kinetic energy of the block as it stops slipping past the belt.
(c) Find the work done in this frame by the external force holding the belt. [6]
Ans. (a) 200 J (b) 200 J (c) 400 J
36. Figure shows a cylindrical tube of length 30 cm which is partitioned by a tight-fitting separator. The separator is very weakly conducing and can freely slide along the tube. Ideal gases are filled in the two parts of the vessel. In the beginning, the temperature in the parts A and B are 400 K and 100 K respectively. The separator slides to a momentary equilibrium position shown in the figure. Find the final equilibrium position of the separator, reached after a long time.
Ans. 10 cm from the left end
39. Show that the internal energy of the air (treated as an ideal gas) contained in a room remains constant as the temperature changes between day and night. Assume that the atmospheric pressure around remains constant and the air in the room maintains this pressure by communicating with the surrounding through the windows etc.
40. Figure shows a cylindrical tube of radius 5 cm and length 20 cm. It is closed by a tight-fitting cork. The friction coefficient between the cork and the tube is 0.20. The tube contains an ideal gas at a pressure of 1 atm and a temperature of 300 K. The tube is slowly heated and it is found that the cork pops out when the temperature reaches 600 K. Let dN denote the magnitude of the normal contact force exerted by a small length dl of the cork along the periphery (see the figure). Assuming that the temperature of the gas is uniform at any instant, calculate dN/dl.
.JPG)
.JPG)
Ans. 1.25 × 10 4 N/m
41. Figure shows a cylindrical tube of cross-sectional area A fitted with two frictionless pistons. The pistons are connected to each other by a metallic wire. Initially, the temperature of the gas is T0 and its pressure is p0 which equals the atmospheric pressure. (a) What is the tension in the wire? (b) What will be the tension if the temperature is increased to 2T0?
Ans. (a) zero (b) p0A
42. Figure shows a large closed cylindrical tank containing water. Initially the air trapped above the water surface has a height h0 and pressure 2p0 where p0 is the atmospheric pressure. There is a hole in the wall of the tank at a depth h1 below the top from which water comes out. A long vertical tube is connected as shown. (a) Find the height h2 of the water in the long tube above the top initially. (b) Find the speed with which water comes out of the hole. (c) Find the height of the water in the long tube above the top when the water stops coming out of the hole.
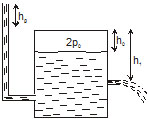
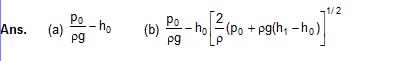
43. An ideal gas is kept in a long cylindrical vessel fitted with a frictionless piston of cross-sectional area 10 cm2 and weight 1 kg (figure). The vessel itself is kept in a big chamber containing air at atmospheric pressure 100 kPa. The length of the gas column is 20 cm. If the chamber is now completely evacuated by an exhaust pump, what will be the length of the gas column? Assume the temperature to remain constant throughout the process.
Ans. 2.2 m
44. An ideal gas is kept in a long cylindrical vessel fitted with a frictionless piston of cross-sectional area 10 cm2 and weight 1 kg. The length of the gas column in the vessel is 20 cm. The atmospheric pressure is 100 kPa. The vessel is now taken into a spaceship revolving round the earth as a satellite. The air pressure in the spaceship is maintained at 100 kPa. Find the length of the gas column in the cylinder.
Ans. 22 cm
45. Two glass bulbs of equal volume are connected by a narrow tube and are filled with a gas at 0ºC at a pressure of 76 cm of mercury. One of the bulbs is then placed in melting ice and the other is placed in a water both maintained at 62ºC. What is the new value of the pressure inside the bulbs? The volume of the connecting tube is negligible.
Ans. 84 cm of mercury
46. The weather report reads. "Temperature 20ºC; Relative humidity 100%. What is the dew point?
Ans. 20ºC
47. The condition of air in a closed room is described as follows. Temperature = 25ºC, relative humidity = 60%, pressure = 104 kPa. If all the water vapour is removed from the room without changing the temperature, what will be the new pressure? The saturation vapour pressure at 25ºC = 3.2 kPa.
Ans. 102 kPa
48. The temperature and the dew point in an open room are 20ºC and 10ºC. If the room temperature drops to 15ºC, what will be the new dew point?
Ans. 10ºC
49. Pure water vapour is trapped in a vessel of volume 10 cm3. The relative humidity is 40%. The vapour is compressed slowly and isothermally. Find the volume of the vapour at which it will start condensing.
Ans. 4.0 cm3
50. A barometer tube is 80 cm long (above the mercury reservoir). It reads 76 cm on a particular day. A small amount of water is introduced in the tube and the reading drops to 75.4 cm. Find the relative humidity in the space above the mercury column if the saturation vapour pressure at the room temperature is 1.0 cm.
Ans. 60%
51. Using figure of the text, find the boiling point of methyl alcohol at 1 atm (760 mm of mercury) and at 0.5 atm.
Ans. 65ºC, 48ºC
52. The human body has an average temperature of 98ºCF. Assume that the vapour pressure of the blood in the veins behaves like that of pure water. Find the minimum atmospheric pressure which is necessary to prevent the blood from boiling. Use figure of the text for the vapour pressures.
Ans. 50 mm of mercury
53. A glass contains some water at room temperature 20ºC. Refrigerated water is added to it slowly. When the temperature of the glass reaches 10ºC, small droplets condense on the outer surface. Calculate the relative humidity in the room. The boiling point of water at a pressure of 17.5 mm of mercury is 20ºC and at 8.9 mm of mercury it is 10ºC.
Ans. 51%
54. 50 m3 of saturated vapour is cooled down from 30ºC to 20ºC. Find the mass of the water condensed. The absolute humidity of saturated water vapour is 30 g/m3 at 30ºC and 16 g/m3 at 20ºC.
Ans. 700 g
55. A barometer correctly reads the atmospheric pressure as 76 cm of mercury. Water droplets are slowly introduced into the barometer tube by a dropper. The height of the mercury column first decreases and then becomes constant. If saturation vapour pressure at the atmospheric temperature is 0.80 cm of mercury, find the height of the mercury column when it reaches its minimum value.
Ans. 75.2 cm
56. 50 cc of oxygen is collected in an inverted gas jar over water. The atmospheric pressure is 99.4 kPa and the room temperature is 27ºC. The water level in the jar is same as the level outside. The saturation vapour pressure at 27ºC is 3.4 kPa. Calculate the number of moles of oxygen collected in the jar.
Ans. 1.93 × 10–3
57. A faulty barometer contains certain amount of air and saturated water vapour. It reads 74.0 cm when the atmospheric pressure is 76.0 cm of mercury and reads 72.10 cm when the atmospheric pressure is 74.0 cm of mercury. Saturation vapour pressure at the air temperature = 1.0 cm of mercury. Find the length of the barometer tube above the mercury level in the reservoir.
Ans. 1.93 × 10–3
58. On a winter day, the outside temperature is 0º and relative humidity 40%. The air from outside comes into a room and is heated to 20ºC. What is the relative humidity in the room? The saturation vapour pressure at 0ºC is 4.6 mm of mercury and at 20ºC it is 18 mm of mercury.
Ans. 9.5%
59. The temperature and humidity of air are 27ºC and 50% on a particular day. Calculate the amount of vapour that should be added to 1 cubic metre of air to saturate it. The saturation vapour pressure at 27ºC = 3600 Pa.
Ans. 13 g
60. The temperature and relative humidity in a room are 300 K and 20% respectively. The volume of the room is 50 m3. The saturation vapour pressure at 300 K is 3.3 kPa. Calculate the mass of the water vapour present in the room.
Ans. 238 g
61. The temperature and the relative humidity are 300 K and 20% in a room of volume 50 m3. The floor is washed with water, 500 g of water sticking on the floor. Assuming no communication with the surrounding, find the relative humidity when the floor dries. The changes in temperature and pressure may be neglected. Saturation vapour pressure at 300 K = 3.3 kPa.
Ans. 62%
62. A bucket full of water is placed in a room at 15ºC with initial relative humidity 40%. The volume of the room is 50 m3. (a) How much water will evaporate? (b) If the room temperature is increased by 5ºC how much more water will evaporate? The saturation vapour pressure of water at 15ºC and 20ºC are 1.6 kPa and 2.4 kPa respectively.
Ans. (a) 361 g (b) 296 g