Worked Out Examples
7. The half-life of 198Au is 2.7 days. Calculate (a) the decay constant, (b) the average-life and
(c) the activity of 1.00 mg of 198Au. Take atomic weight of 198Au to be 198 g/mol.
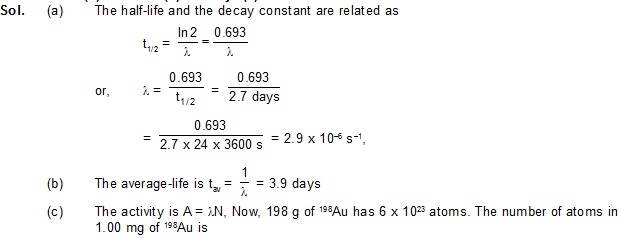
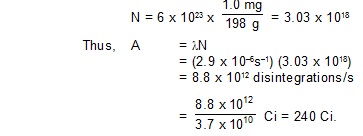
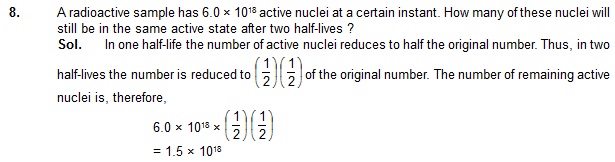
Exercise
Mass of proton mp = 1.007276 u , Mass of atom = 1.007825 u ,
![]()
MeV/C2
1. Assume that the mass of a nucleus is approximately given by M = Amp where A is the
mass number. Estimate the density of matter in k/g m 3 inside a nucleus .
What is the specific gravity of numlear matter ?
Ans: 3 × 10 17 kg/m 3 , 3 × 10 14
2. A neutron star has a density equal to that of the nuclear matter. Assuming the star
to be spherical fine the radius of a neutron star whose mass is 4.0 × 10 30
kg (twice the mass of the sun)
Ans: 15 km
3. Calculate the mass of an alpha particle Its binding energy is 28.2 Me.V
Ans: 4.0016 u
![]()
Ans: 17.34 Me V
5. ![]()
Ans: 7.94 MeV
6. (a) Calculate the energy released if 238 U emits an a-particle. (b) Calculate the energy
to be supplied to 238 U if two protons and two neutrons are to be emitted one by one
The atomic masses of 238 U , 238 Th and 4He are 238.0508 u , 234 .04363 u and
4.00260 u respectively
Ans: (a) 4.255 Me V (b) 24.03 Me V

Ans: 31.65 Me V

10. 32 P beta decays to 32S Find the sum of the energy of the antineutrino and the kinetic
energy of the b- particle Neglect the recoil of the daughter nucleus. Atomic mass of 32
P = 31.974 u and that of 32S = 31.972 u.
Ans: 1.86 Me V
11. A free neutron beta-decays to a proton with a half life of 14 minutes
(a) What is the decay constant?(b) Find the energy liberated in the process.
Ans: (a) 8.25 × 10 – 4 s – 1 (b) 782 Ke V

0.650 Me V (a) What is the energy of the neutron which was emitted together with a positron
of kinetic energy 0.150 Me V ? (b) what is the momentum of this neutrino in kg - m /s ?
Use the formula applicable to photon
Ans: (a) 500 Ke V (b) 2.67 × 10 – 22 kg/ – m/s
14. Potassium -40 can decay in three modes It can decay by beta – emission beta + emission or electron capture

![]()
15. Lithium (Z = 3) has two stable isotopes 6Li and 7Li .When neutrons are bombarded on lithium sample
electrons and a-particles are ejected. Write down the nuclear processes taking place.
 .
.
Atomic mass of 238Th is 228.028726 u That of 224 Ra is 224.0200196 u and that of is 4.00260 u
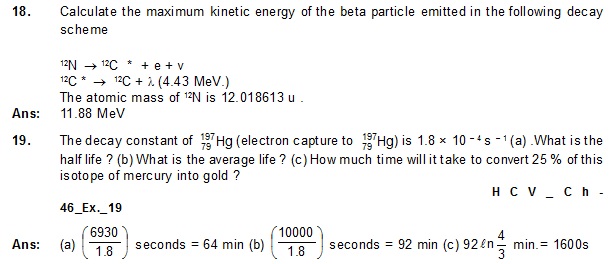
20. The half-life of 198Au is 2.7 days. (a) Find the activity of a sample containing 1.00 µg of 198 Au
(b) What will be the activity after 7 days ? Take the atomic weight of 198 Au to be 198 g/mol.
Ans: (a) 0.244 Ci (b) 0.040
21. Radioactive 131 I has a half-life of 8.0 days A sample containing 131 I has activity 20 µ Ci at t = 0
(a) What is its activity at t = 4.0 days ? (b) What is its decays constant at t = 4.0
Ans: (a) 14 µ Ci (b) 1.4 × 10 – 6 s– 1
22. The decay constant of 238U is 4.9 × 10 – 18 s – 1 (a) What is the average life of 238U ?
(b) What is the half-life of 238U ? (c) By what factor does the activity of a 238 U
sample decrease in 9 × 109 years?
Ans: (a) 6.49 × 109 y (b) 4.5 × 10 9 y (c) 4
23. A certain sample of a radioactive material decays at the rate of 500 per second at a certain time
The count rate falls to 200 per second after 50 minutes (a) What is the dacey constant of the sample?
(b) What is its half-life ?
Ans: 3.05 × 10 – 4 s (b) 38 min
24. The count rate from a radioactive sample falls from 4.0 × 10 6 per seconds to 1.0 × 10 6 per second
in 20 hours what will be the count rate 100 hours after the beginning ?
Ans: 3.9 × 10 3 per second
25. The half-life of 226Ra is 1602 y .Calculate the activity of 0.1 g of RaCl2 in which all the radium in
the from of 220Ra Taken atomic weight of Ra to be 226 g/mol and that of Cl to be 35.5 g/mol
Ans: 2.8 × 10 9 disintergrations/s
26. The half-life of a radioisotope is 10 h .Find the total number of disintegrations in the tenth
hour measured from a time when the activity was 1 Ci.
Ans: 6.91 × 10 13
27. The selling rate of a radioactive isotope is decided by its activity what will be the second hand
rate of a one month old 32 P(t1/2 = 14.3 days ) source if it was originally purchased for 800 rupees ?
Ans: 187 rupees
28. 57Co dacys to 57 Fe by beta+ - emission The resulting 57 Fe is in its excited state and comes to the
ground state by emitting state and comes to the ground state by emitting g -rays emission is 10 – s
A sample of 57 Co gives 5.0 ×109 gamma rays per second. How much time will elapse before the
emission rate of gamma rays drops to 2.5 × 10 9 per second ?
Ans: 270 days
29. Carbon (Z = 6 ) with mass number 11 decays to boron (Z = 5 ) (a) Is it a b decay or b decay ?
(b) The half-life of the decay scheme is 20.3 minutes .How much time will elapse before a
mixture of 90% carbon 11 and 10% boron -11 (by the number of atoms ) converts itself into
a mixture of 10% carbon-11 and 90% boron-11?
Ans : (a) b + (b) 64 min
30. 4 × 1023 tritium atoms are contained in a vessel. The half-life of decay of tritium nuclei is
12.3 Find (a) the activity of the sample ,(b) the number of decays in the next 10 hours
(c) the number of decays in the next 6.15 y.
Ans : (a) 7.146 × 10 14 disintergrations/s
31. A point source emitting alpha particles is placed at a distance of 1m from a counter which
records any alpha particle falling on its 1cm 2 window .If the source contains 6.0 × 1016
active nuclei and the counter records a rate of 50000 count /second find the decay constant
Assume that the source emits alpha particles fall nearly in all directions and the alpha
particles fall nearly normally on the window.
Ans: 1.05 × 10 – 7 s –1
32. 238U decays to 20Pb with a half-life of 4.47 × 109 y. This happens in a number of steps Can
you justify a single half-life for this chain of processes? A sample of rock is found to contain
2.00 mg of 238U and 0.600 mg of 20b Pbeta Assuming that all the lead has come from
uranium find the life of the rock.
Ans: 1.92 × 10 9 y
33. When charcoal is prepared from a living tree, it shows a disintergration rate of 15 .3
disintergrations of 14C per gram per minute A sample from an ancient piece of charcoal
show 14C activity to be 12.3 disintergrations per gram per minute How old is this sample?
Half-life of 14C is 5730y.
Ans : 1800 y
34. 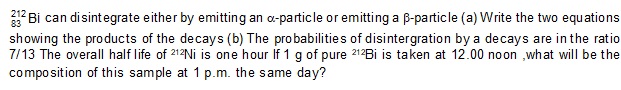
Ans: about 83 years go
35. The count rate of nuclear radiation coming from a radioactive sample containing 128I varies
with time as follows .
Time t ( minute ): 0 25 50 75 100
Count rate R (109 s– 1) : 30 16 8.0 3.8 2.0
(a) Plot in (Ro/R ) against t (b) From the slope of the best straight line through the points find
the decays constant l (c) Calculate the half-life t 1/2.
Ans: (b) 0.028 min – 1 approx (c) 25 min approx
36. The half-life of 40K is 1.30 × 10 9 y A sample of 1.00g of pure KCI gives 160 counts/s
Calculate the relative abundance of 40K (fraction of 40K present ) in natural potassium.
Ans: 0.12%
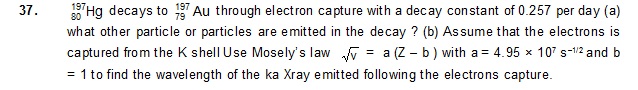
Ans: (a) neutrino (b) 20 pm
38. A radioactive isotope is being produced at a constant rate dN/dt = R in an experiment .
The isotope has a half-life t1/2. Show that after a time t >> t1/2, the value of this constant.

40. In an agricultural experiment ,a solution containing 1 mole a of radioactive material (t1/2 = 14.3 days )
was injected into the roots of a plant .The plant was allowed 70 hours to settle down and then activity
was measured in its fruit If the activity measured was 1 µ Ci what per cent of activity is transmitted
from the root to the fruit in steady state ?
Ans : 1.26 × 10 – 11 %
41. A vessel of volume 125 cm3 contains tritium (3H t1/2 = 12.3 y ) at 500 kpa and 300 K Calculate
the activity of the gas
Ans : 724 Ci
42. 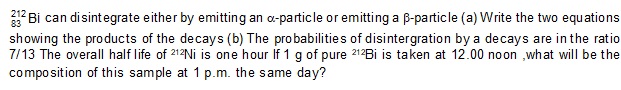
![]()
43. A sample contains a mixture of 108 Ag and 110Ag isotopes each having an activity of 8.0 × 108
disintergrations per second .110 Ag is known to have larger half-life than 108Ag. The activity A
is measured as a function of time and the following data are obtained.
(a) Plot In (A/Ao) versus time (b) See that for large values of time the plot is nearly linear Deduce
the half-life of 110Ag from this portion of the plot (c)Use the half-life of 110 Ag to calculate the
activity corresponding to 108 Ag in the first 50 S (d) plot in (A/Ao) versus time for 108 Ag for the
first 50 s. (e) Find the half-life of 108 Ag
Ans : the half life of 110 Ag 24.4 s and of 108 Ag = 144s
44. A human body excretes (removes by waste discharge sweating etc.) certain materials by a low
similar to radioactivity If technetium is injected in some form in a human body , the excretes
half the amount in 24 hours A patient is given an injection containing 99 Tc this isotope is
radioactive with a half -life of 76 hours The activity from the body just after the injection
is 6 µ Ci How much time will elapse before the activity falls to 3 µ Ci ?
Ans : 4.8 hours
45. A charged capacitor of capacitance C is discharged through a resistance R A radioactive
sample decays with an average life t Find the value of R for which the ratio of the electrostatic
field energy stored in the capacitor to the activity of the radioactive sample remains constant in time.
Ans : 2 t / C
46. Radioactive isotopes are produced in a nuclear physics experiment at a constant rate dN/dt = An
inductor of inductance 100 mH a resistor of resistance 100ohm and a battery are connected to from
a series circuit .The circuit is switched on at the instant the production of radioactive isotope starts
It is found that i/N remains constant in time where i is the current in the circuit at time t and N is
the number of active nuclei at time t Find the half-life of the isotope.
Ans: 6.93 × 10 4 K
47. Calculate the energy released by 1 g of natural uranium assuming 200 Me V is released in
each fission event and that the fissioable 235 U has an abundance of 0.7 % by weight in
natural uranium
Ans: 5.7 × 10 8 J
48. A uranium reactor dipoles thermal energy at a rate of 300 MW Calculate the amount
of 235 U being consumed every second . Average energy released per fission is 200 Me V.
Ans: 3.7 mg
49. A town has a population of 1 million .The average electric power needed per person is 300 W.
A reactor is to be designed to supply power is converted into electric with which thermal
power is converted into electric power is aimed at 25% (a) Assuming 200 MeV of thermal
energy to come from each fission event on an average find The number of events that should
take place energy day (b) Assuming the fission to take place largely through 235 U at what
rate will the amount of 235 U decrease ? Express your answer in kg/day (c) Assuming that
uranium enriched to 3% in 235 U will be used how much uranium is needed per month (30 days )?
Ans: (a) 3.24 × 10 24 (b) 1.26 kg /day (c) 1263 kg)
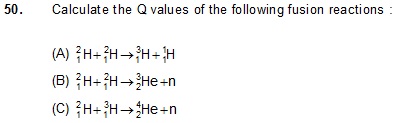
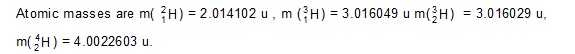
Ans: (a) 4.05 Me V (b) 3.25 Me V (c) 17.57 Me V
51. Consider the fusion in helium plasma. Find the temperature at which the average
thermal energy 1.5 kT equals the Coulomb potential energy at 2 fm.
Ans : 2.23 × 10 10 K
52. Calculate the Q -value of the fusion reaction
4He + 4He = 8Be.
Is such a fusion energetically favorable? Atomic mass of 8Be is 8.0053 u and that of 4He is 4.0026 u.
Ans: – 93 .1 Ke V , no
