Solved Examples
1. Calculate the radius of 70Ge.
Sol. We have,
R = R0 A1/3 = (1.1 fm) (70)1/3
= (1.1 fm) (4.12) = 4.53 fm.
2. Calculate the binding energy of an alpha particle from the following data:

Take 1 u = 931 MeV/c2.
Sol. The alpha particle contains 2 protons and 2 nutrons. The binding energy is
B = (2 × 1.007825 u + 2 × 1.008665 u – 4.00260 u)c2
= (0.03038 u)c2
= 0.03038 × 931 MeV = 28.3 MeV.
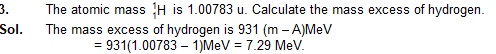
Sol. The mass excess of hydrogen is 931 (m – A)MeV
= 931(1.00783 – 1)MeV = 7.29 MeV.
4. The decay constant for the radioactive nuclide 64Cu is 1.516 × 10–5 s–1.
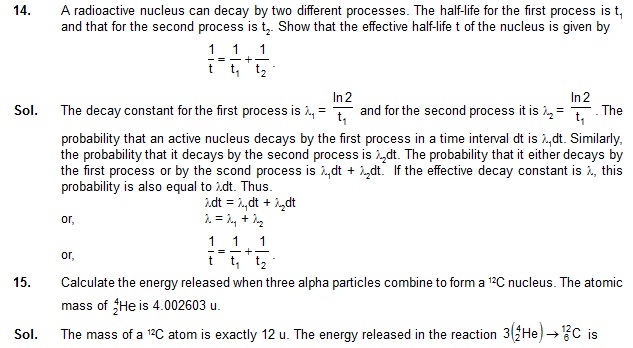
Find the activity of a sample containing 1 mg of 64Cu. Atomic weight of copper = 63.5 g/mole.
Neglect the mass difference between the given radioisotope and normal copper.
Sol.
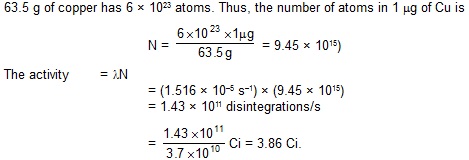
5. The half-life of a radioactive nuclide is 20 hours. What fraction of original activity will remain after 40 hours?
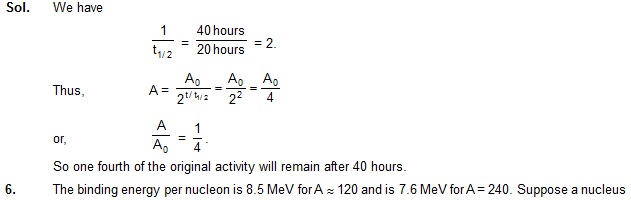
with A = 240 breaks into two nuclei of nearly equal mass numbers. Calculate the energy released in the process.
Sol. Suppose the heavy nucleus had Z protons and N neutrons. The rest mass energy of this nucleus would be
E = Mc2 = (Zmp + Nmn)c2 – B1
= (Zmp + Nmn)c2 – 7.6 × 240 MeV.
If there are Z1 protons and N1 neutrons in the first fragment, its rest mass energy will be
E1 = M1c2 = (Z1mp + N1mn)c2 – B2
= (Z1mp + N1mn)c2 – (8.5 MeV) (Z1 + N1).
Similarly, if there are Z2 protons and N2 neutrons in the first fragment, its rest mass energy will be
E2 = (Z2mp + N2mn)c2 – (8.5 MeV) (Z2 + N2).
The energy released due to the breaking is
E – (E1 + E2)
= [(Z – Z1 – Z2]mp c2 + (N – N1 – N2)mnc2] + [(Z1 + Z2 + N1 + N2) × 8.5 – 240 × 7.6] MeV
= 240 × (8.5 – 7.6) MeV = 216 MeV.
We have used the fact that Z1 + Z2 = Z, N1 + N2 = N and Z1 + Z2 + N1 + N2 = Z + N = 240.
Thus, 216 MeV of energy will be released when this nucleus breaks.
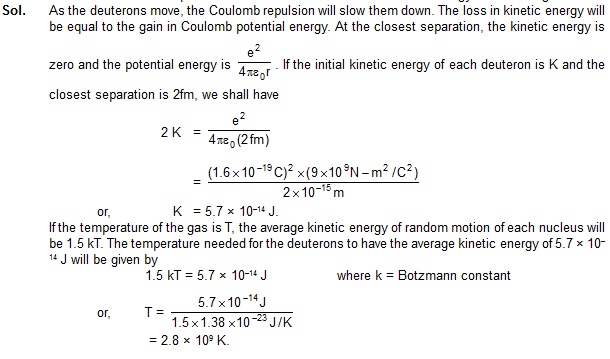
7. Consider two deuterons moving towards each other with equal speeds in a deutron gas.
What should be their kinetic energies (when they are widely separated) so that the closest
separation between them becomes 2fm? Assume that the nuclear force is not effective for
separations greater than 2 fm. At what temperature will the deuterons have this kinetic energy on an average?
Workd out Examples
1. Calculate the electric potential energy due to the electric repulsion between two nuclei
of 12C when they ‘touch’ each other at the surface
Sol. The radius of a 12C nucleus is
R = R0 A1/3
= (1.1 fm) (12)1/3 = 2.52 fm.
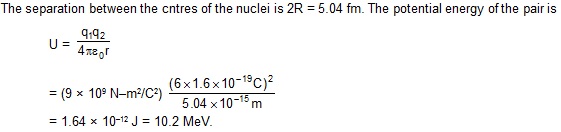
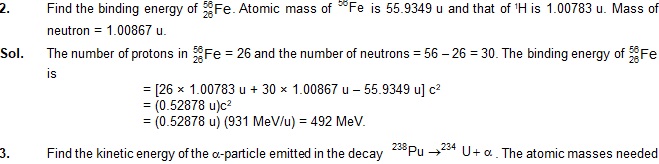
are as follows :
238Pu 234U 4He
238.04955 u 234.04095 u 4.002603 u
Neglect any recoil of the residual nucleus.
Sol. Using energy conservation,
m(238Pu)c2 = m (234U)c2 + m(4He)c2 + K
or, K = [m(238Pu) – m(234U) – m(4He)]c2
= [238.04955 u – 234.04095 u – 4.002603 u] (931 Me V/u)
= 5.58 MeV.
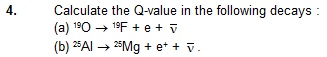
The atomic masses needed are as follows:
19O 19F 25Al 25Mg
19.003576 u 18.998403 u 24.990432 u 24.985839 u
Sol. (a) The Q-value of beta– -decay is
Q = [m(19O) – m(19F)]c2
= [19.003576 u – 18.998403 u ] (931 MeV/u)
= 4.816 MeV
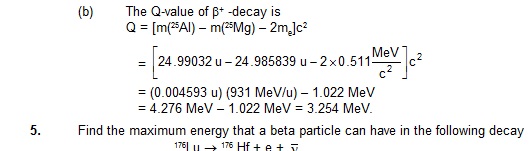
Atomic mass of 176Lu is 175.942694 u and that of 176Hf is 175.941420 u.
Sol. The kinetic energy available for the beta particle and the antineutrino is
Q = [m(176Lu) – m (176Hf)]c2
= (175.942694 u – 175.941420 u) (931 MeV/u)
= 1.182 MeV.
This energy is shared by the beta particle and the antineutrino. The maximum kinetic energy
of a beta particle in this decay is, therefore, 1.182 MeV when the antineutrino practically does not get any share.
![]()
where 198Hg* represents a mercury nucleus in an excited state at energy 1.088 MeV above the ground state.
What can be the maximum kinetic energy of the electron emitted? The atomic mass 198Au is 197.968233
u and that of 198Hg is 197.966760 u.
Sol. If the product nucleus 198Hg is formed in its ground state, the kinetic energy available to the electron and the antineutrino is
Q = [m(198Au) – m(198Hg)]c2 .
As 198Hg* has energy 1.088 MeV more than 198Hg in ground state, the kinetic energy actually available is
Q = [m(198Au) – m(198Hg)]c2 – 1.088 MeV
![]()
= 1.3686 MeV – 1.088 MeV = 0.2806 MeV.
This is also the maximum possible kinetic energy of the electron emitted.
7. The half-life of 198Au is 2.7 days. Calculate (a) the decay constant, (b) the average-life and
(c) the activity of 1.00 mg of 198Au. Take atomic weight of 198Au to be 198 g/mol.
198Au dk v)Z&vk;qdky 2.7 fnu gSA crkb;s (a) {k; fu;rkad (b) vkSlr vk;q
(c) 1.00 mg 198Au dh lfØ;rkA 198Au dk ijek.kq Hkkj 198 g/mol yhft,A
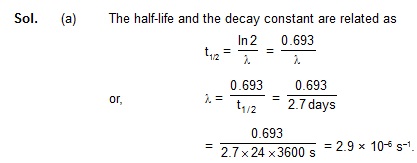
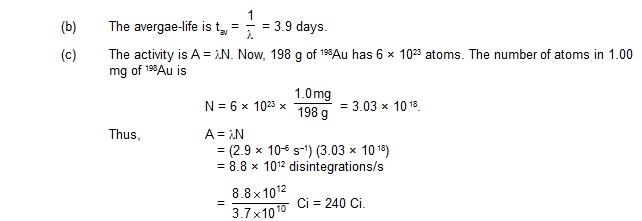
8. A radiactive sample has 6.0 × 1018 active nuclei at a certain instant. How many of these nuclei
will still be in the same active state after two half-lives?
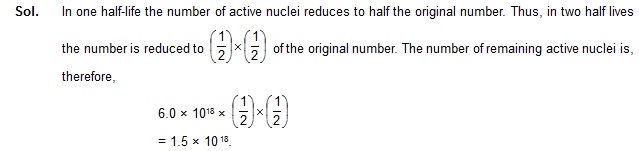
9. The activity of a radioactive sample falls from 600 s–1 to 500 s–1 in 40 minutes. Calculate its half-life.
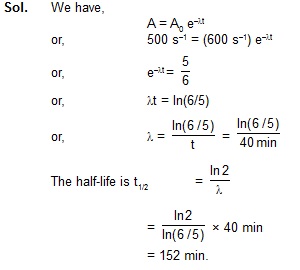
10. The number of 238U atoms in an ancient rock equals the number of 206Pb atoms. The half-life of decay
of 238U is 4.5 × 10 9 y. Estimate the age of the rock assuming that all the 206Pb atoms are
formed from the decay of 238U.
Sol. Since the number of 206Pb atoms equals the number of 238U atoms, half of the original 238U atoms have decayed.
It takes one half-life to decay half of the active nuclei. Thus, the sample is 4.5 × 109 y old.
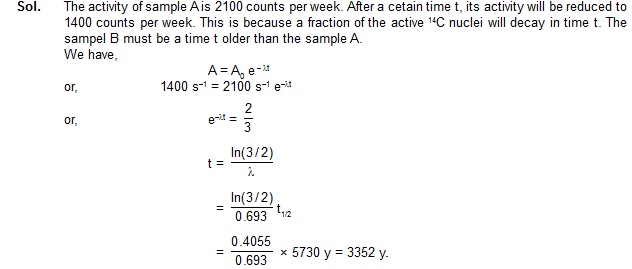
11. Equal masses of two samples of charcoal A and B are burnt separately and the resulting carbon dioxide are
collected in two vessels. The radioactivity of 14C is measured for both the gas samples. The gas from the
charcoal A gives 2100 counts per weak and the gas from the charcoal B gives 1400 counts per week.
Find the age difference between the two samples. Half-life of 14C = 5730 y.
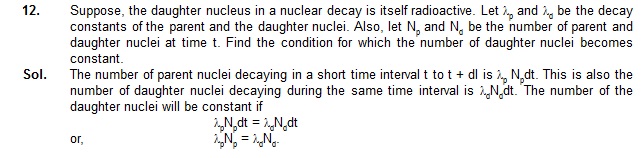
13. A radioactive sample decays with an avergae-life of 20 ms. A capacitor of capcitance 100 mF is
charged to some potential and then the plates are connected through a resistance R. What should be the
value of R so that the ratio of the charge on the capacitor to the activity of the radioactive sample
remains constant in time?
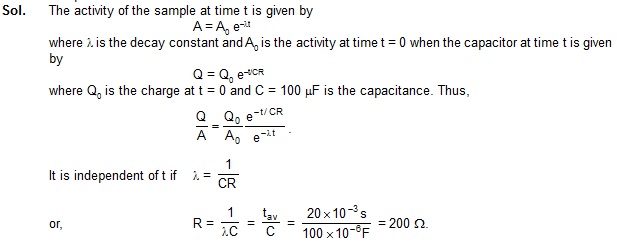
![]()
Question for short answer
1. If neutrons exert only attractive force, why don’t we have a nucleus containing neutrons alone ?
2. Consider two pairs of neutrons. In each pair, the separation between the neutrons is the same.
Can the force between the neutrons have different magnitudes for the two pairs ?
3. A molecule of hydrogen contains two protons and two electrons. The nuclear force between
these two protons is always neglected while discussing the behaviour of a hydrogen molecule. Why ?
4. Is it easier to take out a nucleon from carbon or from iron ? From iron or from lead ?
5. Suppose we have 12 protons and 12 neutrons. We can assemble them to from either a 24Mg
nucleus or two 12C nuclei. In which of the two cases more energy will be liberated ?
6. What is the difference between cathode rays and beta rays ? When the two are travelling
in space, can you make out which is the cathode ray and which is the beta ray ?
7. If the nucleons of a nucleus are separated from each other, the total mass is increased,
Where does this mass come from ?
8. In beta decay, an electron (or a positron) is emitted by a nucleus. Does the remaining
atom get oppositely charged ?
9. ![]()
10. Does a nucleus lose mass when it suffers gamma decay ?
11. In a typical fission reaction, the nucleus is split into two middle-weight nuclei of unequal masses.
Which of the two (heavier or lighter) has greater kinetic energy ? Greater linear momentum ?
12. If three helium nuclei combine to form a carbon nucleus, energy is liberated. Why can’t
helium nuclei combine on their own and minimise the energy ?
Objective - I
1. The mass of a netural carbon atom in ground state is
(A) exact 12 u (B) less than 12 u (C) more than 12 u
(D) depends on the from of carbon such as graphite or charcoal.
2. The mass number of a nucleus is equal to
(A) the number of neutrons in the nucleus (B) the number of protons in the nucleus
(C) the number of protons in the nucleus (D) none of them
3. As compared to 12C atom, 14C atom has
(A) two extra protons and two extra electrons (B) two extra protons but no extra electron
(C) two extra neutorns and no extra electrons (D) two extra neutons and two extra electrons
4. The mass number of a nucleus is
(A) always less than its atomic number
(B) always more than its atomic number
(C) equal to its atomic number
(D*) sometimes more than and sometimes equal to its atomic number
5. The graph of ln (R/R0) versus In A(R=radius of a nucleus and A = its mass number) is
(A) a straight line (B) a parabola (C) an ellipse (D) none of them
6. Let Fpp, Fpn and Fnn denote the magnitudes of the nuclear force by a proton on a proton, by
a proton on a neutron and by a neutron on a neutron respectively. When the separation is 1 fm,
(A) Fpp> Fpn = Fnn (B*) Fpp= Fpn = Fnn (C) Fpp> Fpn > Fnn (D) Fpp< Fpn = Fnn
7. Let Fpp, Fpn and Fnn denote the magnitudes of the net force by a proton on a proton by a
proton on a neutron and by a neutron on a neutron respectively. Neglect gravitational force.
When the separation is 1 fm,
(A) Fpp> Fpn = Fnn (B) Fpp= Fpn = Fnn (C) Fpp> Fpn > Fnn (D*) Fpp< Fpn = Fnn
8. Two protons are kept at a separation of 10 nm. Let Fn and Fe be the nuclear force and
the electromagnetic force between them.
(A) Fe = Fn (B*) Fe >> Fn (C) Fe << Fn (D) Fe o Fn
9. As the mass number A increases, the binding energy per nucleon in a nucleus
(A) increases (B) decreases (C) remains the same
(D) varies in a way the depends on the actual value of A.
10. Which of the following is a wrong description of binding energy of a nucleus ?
(A) It is the energy required to break a nucleus into its constituent nucleons.
(B) It is the energy mad avilable when free nucleous combine to from a nucleus
(C) It is the sum of the rest mass energies of its nucleous minus the rest mass energy of the nucleus
(D) It is the sum of the kinetic energy of all the nucleous in the nucleus
11. In one average-life
(A) half the active nuclei decay (B) less than half the active nuclei decay
(C) more than half the active nuclei decay (D) all the nuclei decay
12. In a radioactive decay, neither the atomic number nor the mass number changes.
Which of the following particles is emitted in the decay ?
(A) proton (B) neutorn (C) electron (D) photon
13. During a negative beta decay,
(A) an atomic electron is ejected
(B) an electron which is already present within the nucleus is ejected
(C) a neutron in the nucleus decays emitted an electron
(D) a proton in the nucleus decays emitting
14. A freshly prepared radiocative source of half-life 2 h emits radiation of intensity which is
64 times the permissible safe level. The minimum time after which it would be possible
to work safely with this source is -
(A) 6 h (B) 12 h (C) 24 h (D) 128 h
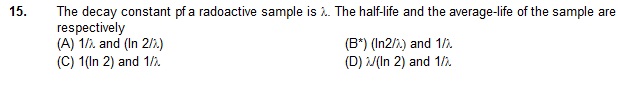
16. An a-particle is bombarded on 14N. As a result, a 17O nucleus is formed and a particle is emitted.
This particle is alpha -
(A) neutron (B) proton (C) electron (D) positron
17. Ten grams of 57Co kept in an open container beta-decays with a half-life of 270 days.
The weight of the material inside the container after 540 days will be very nearly
(A) 10 g (B) 5 g (C) 2.5 g (D) 1.25 g
18. Free 238U nuclei kept in a train emit alpha particles. When the train is stationery and a uranium
nucleus decays, a passenger measures that the separation between the alpha particle and the recoiling
nucleus beomes x in time t after the decay. If a decay takes place when the train is moving at a
uniform speed u, the distance between the alpha particle and the recoiling nucleus at a time t
after the decay, as measured by the passenger will be
(A) x + ut (B) x - ut (C*) x (D) depends on the direction of the train
19. During a nuclear fission reaction,
(A) a heavy nucleus breaks into two fragments by itself
(B) a light nucleus bombarded by thermal neutrons break up
(C*) a heavy nucleus bombarded by thermal neutrons breaks up
(D) two light nuclei combine to give a heavier nucleus and possibly other products.
Objective - II
1. As the mass number A increases, which of the following quantities related toa nucleus do not change ?
(A) mass (B) vloume (C*) density (D) binding energy
2. The heavier nuclei tend to have larger N/Z ration becaues
(A) a neutron is heavier than a proton
(B) a neutron is an unstabel particle
(C*) a neutron does not exert electric repulsion
(D*) Coulomb forces have longer range compared to nuclear forces
3. A free neutron decays to a proton but a free proton does not decay to a neutron. This is beacuse
(A) neutron is a composite particle made of a proton and an electron whereas proton is fundamental particle
(B) neutron is an uncharged particle whereas proton is a charged particle
(C*) neutron has larger rest mass than the proton
(D) weak forces can operate in a neutron but not in a proton.
4. Consider a sample of a pure beta-active material
(A) All the beta particles emitted have the same energy
(B) The beta particles originally exist inside the nucleus and are ejected at the time of beta decay
(C) The antineutrino emitted in a beta decay has zero mass and hence zero momentum.
(D*) The active nucleus changes to one of its isobars after the beta decay
5. In which of the following decays the element does not change ?
(A) alpha-decay (B) beta+decay (C) beta--decay (D*) lambda-decay
6. In which of the follwoing decaus the atomic number decreases ?
(A*) alpha-decay (B*) beta+decay (C) beta– decay (D) lambda-decay
7. Magnetic field does not cause deflection in
(A) alpha-rays (B) beta-plus rays (C) beta-minus rays (D*) gamma rays
8. Which of the follwoing are electromagnetic waves ?
(A) alpha-rays (B) beta-plus rays (C) beta-minus rays (D*) gamma rays
9. Two lithium nuclei in a lithium vapour at room temperature do not combine to form a carbon nucleus because ?
(A) a lithium nucleus is more tightly bound than a carbon nucleus
(B) carbon nucleus is an unstable particle
(C) it is not energetically favourable
(D*) Coulomb repulsion does not allow the nuclei to come very close
10. For nuclei with A > 100
(A) the binding energy of the nucleus decreases on an average as A increases
(B*) the binding energy per nucleus decreases on an average as A increases
(C*) if the nucleus breaks into two roughly equal parts, energy is released
(D) if two nuclei fuse to form a bigger nucleus, energy is released.