Solved Examples
Example 24.1.
Calculate the rms speed of nitrogen at STP (pressure = 1 atm and temperature = 0ºC). The density of nitrogen in these conditions is 1.25 kg/m3.
Sol. At STP, the pressure is 1.0 × 105 N/m2. The rms speed is
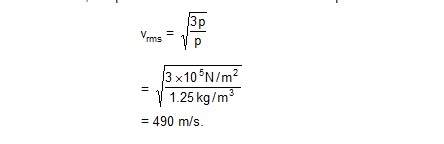
Example 24.2
If the rms speed of nitrogen molecules is 490 m/s at 273 K, find the rms speed of hydrogen molecules at the same temperature.
Sol. The molecular weight of nitrogen is 28 g/mole and that of hydrogen is 2 g/mole. Let m1, m2 be the masses and v1, v2 be the rms speeds of a nitrogen molecule and a hydrogen molecule respectively. Then m1 = 14 m2. Using equation (24.6),
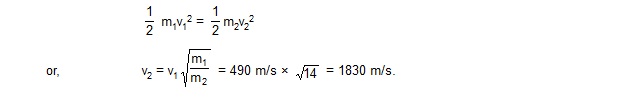
Example 24.3
Calculate the number of molecules in each cubic metre of a gas at 1 atm and 27ºC.
Sol. We gave pV = NkT

Example 24.4
Find the rms speed of oxygen molecules in a gas at 300 K.
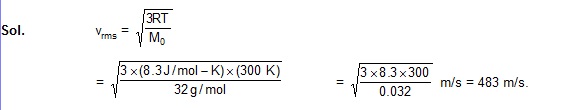
Example 24.5
At what external pressure will water boil at 140ºC? Use table (24.1) for vapour pressure data and express the answer in atm.
Sol. The saturation vapour pressure of water at 140ºC is 2710 mm of Hg. Thus, water will boil at 140ºC at this pressure. Now 760 mm of Hg = 1 atm.
Thus, 2710 mm of Hg = 2710/760 atm = 3.56 atm.
The pressure inside a pressure cooker is of this order when it whistles. So, the temperature inside is of the order of 140ºC which helps in cooking the food much faster.
Example 24.6
The vapour pressure of air at 20ºC is found to be 12 mm of Hg on a particular day. Find the relative humidity. Use the data of table (24.1)
Sol. The saturation vapour pressure of water at 20ºC is 17.5 mm of Hg. Thus, the relative humidity is

Example 24.7
In an experiment with Regnault’s hygrometer, dew appears at 10ºC when the atmospheric temperature is 40ºC. Using table (24.1), find the relative humidity.
Sol. The dew point is 10ºC. The saturation vapour pressure at this temperature is 8.94 mm of Hg from table (24.1). Also, the saturation vapour pressure of air
at 40ºC is 55.1 mm of Hg. The relative humidity expressed in percentage

Questions for Short answer
Objective - I
1. Which of the following parameters is the same for molecules of all gases at a given temperature?
(A) mass (B) speed (C) momentum (D*) kinetic energy
2. A gas behaves more closely as an ideal gas at
(A) low pressure and low temperature (B) low pressure and high temperature
(C) high pressure and low temperature (D) high pressure and high temperature
3. The pressure of an ideal gas is written as P = 2E/3V. Here E refers to
(A) translatioal kinetic energy (B) rotational kinetic energy
(C) vibrational kinetic energy (D) total kinetic energy
4. The energy of a given sample of an ideal gas depends only on its
(A) volume (B) pressure (C) density (D) temperature
5. Which of the following gases has maximum rms speed at a given temperature ?
(A) hydrogen (B) nitrogen (C) oxygen (D) carbon dioxide
6. Fig. shows graphs of pressuer vs. density for an ideal gas at two temperature T1 and T2.
(A) T1 > T2 (B) T1 = T2 (C) T1 < T2 (D) any of the three is possible
7. The mean square speed of the molecules of a gas at absolute temperature T is proportional to
(A) 1/T (B) ÖT (C) T (D) T2
8. Suppose a container is evacuated to leave just one molecule of a gas in it. Let na and nrms represent the average speed and the rms speed of the gas.
(A) va > vrms (B) va < vrms (C) va = vrms (D) vrms is undefined
9. The rms speed of oxygen at room temperature is about 500 m/s. The rms speed of hydrogen at the same temperature is about
(A) 125 m/s (B) 200 m/s (C) 800 m/s (D) 31 m/s
10. The pressure of a gas kept in an isothermal container is 200 kPa. If half the gas is removed from it, the pressure will be
(A) 100 kPa (B) 200 kPa (C) 400 kPa (D) 800 kPa
11. The rms speed of oxygen molecules in a gas is n. If the temperature is doubled and the O2 molecule dissociate into oxygen atoms, the rms speed will become
(A) v (B) v Ö2 (C) 2 v (D) 4v
12. The quantity pV/kT represents
(A) mass of the gas (B) kinetic energy of the gas
(C) number of moles of the gas (D) number of molecules in the gas
13. The process on an ideal gas, shown in fig. is -
(A) isothermal (B) isobaric (C*) isochoric (D) none of these
14. There is some liquid in a closed bottle. The amount of liquid is continuously decreasing. The vapour in the remaining part
(A) must be saturated (B) must be unsaturated
(C) may be unsaturated (D) there will be no vapour
15. There is some liquid in a closed bottle. The amount of liquid is remains constant as time passses. The vapour in the remaining part
(A) must be saturated (B) must be unsaturated
(D) may be unsaturated (D) there will be no vapour
16. Vapour is injected at a uniform rate in a closed vessel which was initially evacuated. The pressure in the vessel
(A) increases continuously (B) decreases continuously
(C) first increases and then decreases (D) first increases and then becomes constant
17. A vessel A has volume V and a vessel B has volume 2V. Both contain some water which has constant volume. The pressure in the space above water is Pa for vessel A and Pb for vessel B :
(A) Pa = Pb (B) Pa = 2Pb (C) Pb = 2Pa (D) Pb = 4Pa
Objective - II
1. Consider a collision between an oxygen molecule and a hydrogen molecules in a misture of oxygen and hudrogen kept at room temperature. Which of the following are possible ?
(A) The kinetic energies of both the molecules increase.
(B) The kinetic energies of both the molecules decrease
(C) The kinetic energy of the oxygen molecule increases and that of the hydrogen molecules decresases.
(D) The kinetic energy of the hydrogen molecules increases and that of the oxyzen molecule decreases.
2. Consider a mixture of oxygen and hydrogen kept at room tempertaure. As compared to a hydrogen molecule an oxygen molecule hits the wall
(A) With greater average speed (B) with smaller average speed
(C) with greater average kinetic energy (D) with smaller average kinetic energy.
3. Which of the following quantities is zero on an average for the molecules of an ideal gas in equilibrium ?
(A) kinetic energy (B) momentum (C) density (D) speed
4. Keeping the number of moles, volume and temperature the same, which of the following are the same for all ideal gas ?
(A) rms speed of a milecule (B) density (C) pressure (D) average magnitude of momentum.
5. The average momentum of a molecule in a sample of an ideal gas depends on
(A) temperature (B) number of moles (C) volume (D) none of these
6. Which of the following quantities is the same for all ideal gases at the same temperature ?
(A) the kinetic energy of 1 mole (B) the kinetic energy of 1 g
(C) the number of molecules in 1 mole (D) the number of molecules in 1 g
7. Consider the quantity MkT / pV of an ideal gas where M is the mass of the gas. It depends on the
(A) temperature of the gas (B) volume of the gas
(C) pressure of the gas (D) nature of the gas
Worked Out Examples
1. A vessel of volume 8.0 x 10 -3 m 3 contains an ideal gas at 300 K and 200 kPa. The gas is allowed to leak till the pressure falls to
125 kPa. Calculate the amount of the gas (in moles) leaked assuming that the temperature remains constant.
Solution : As the gas leaks out, the volume and the temperature of the remaining gas do not change. The number of
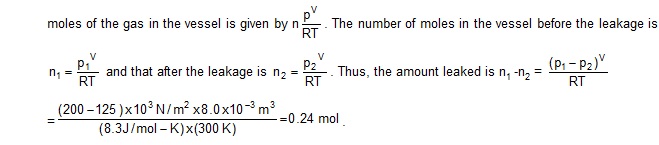
2. A Vessel of volume 2000 cm3 contains 0.1 mole of oxygen and 0.2 mole of carbon dioxide. If the temperature of the mixture is 300 K, find its pressure.
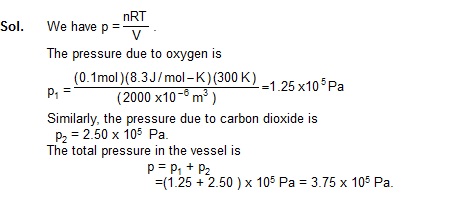
3. A mixture of hydrogen and oxygen has volume 2000 cm3, temperature 300K, pressure 100 kPa and mass 0.76 g. Calculate the masses of hydrogen and oxygen in the mixture.
Sol. : Suppose there aare n1 moles of hydrogen and n2 moles of oxygen in the mixture. The pressure of the mixture will be.
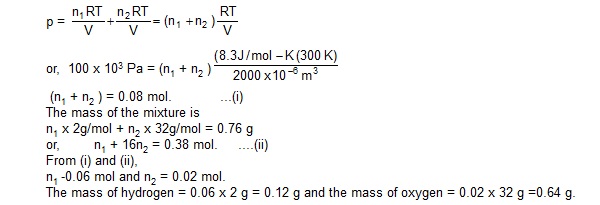
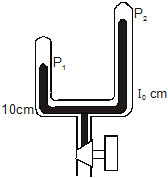
4. A mercury monometer ( figure 24-W1) consits of two unequal arms of equal cross-section 1 cm2 and lengths 100 cm and 50 cm.
The two open ends are sealed with air in the tube at a pressure of 80 cm of mervury. Some amount of mercury is now introduced in
the manometer through the stopcock connected to it. If mercury rises in the shorter tube to a length 10 cm in steady state,
find the length of the mercury column risen in the longer tube.
Sol.: Let p1 and p2 be the pressures in centicetre of mercury in the two arms after introducing mercury in the tube. Suppose
the mercury column rises in the second arm to lo cm.
Using pV = constant for the shorter arm,
(80 cm) (50 cm) = p1 (50 cm – 10 cm)
or, p1 = 100 cm. .... (i)
Using pV = constant for the longer arm,
(80 cm) (100 cm) = p2(100 – l0) cm ....(ii)
From the figure,
p1 = p2 + (l0 – 10)cm
Thus by (i),
100 cm = p2 + (l0 – 10)cm.
or, p2 = 110 cm – l0 cm.
Putting in (ii),
(110 – l0) (100 – l0) = 8000
or, l02 – 210 l0 + 3000 = 0
or, l0 = 15.5.
The required length is 15.5 cm.
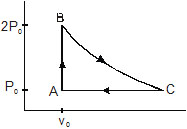
5. An ideal gas has pressure p0, volume V0 and temperature T0. It is taken through an isochoric process till its pressure is doubled.
It is now isothermally expanded to get the original pressure. Finally, the gas is isobarically compressed to its original volume V0.
(a) Show the process on a p–V diagram.
(b) What is the temperature in the isothermal part of the process?
(c) What is the volume at the end of the isothermal part of the process?
Sol. (a) The process is shown in a p–V diagram in figure. The process starts from A and goes through ABCA.
(b) Applying pV = nRT at A and B,
p0V0 = nRT0
and (2p0)V0 = nRTB.
Thus, TB = 2T0
This is the temepratrure in the isothermal part BC.
(c) As the process BC is isothermal, TC = TB = 2T0.
Applying pV = nRT at A and C,
p0V0 = nRT0
and p0VC = nR(2T0)
or, VC = 2V0.
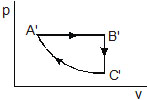
6. A cylic process ABCA shown in the V–T diagram (figure) is performed with a constant mass of an ideal gas. Show the same process on a p–V diagram. In the figure. CA is parallel to the V-axis and BC is parallel to the T-axis.
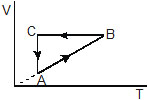
Sol. The p–V diagram is shown in figure. During the part AB of figure, V is proportional to T. Thus, pV/T = nR, we see that the pressure p is constant in this part. This is represented by the part A’B’ in the p-V diagram. During the part, BC, volume is constant. Thus, p/T is constant. As the temperature decreases, pressure also decreases. This is represented by the part B’C’ in the p-V diagram. During the part CA, the temperature remains constant so that pV = constant. Thus, p is inversely proportional to V. This is represented by the port C’A’ in the p-V diagram.
7. Two closed vessels of equal volume contain air at 105 kPa, 300 K and are connected through a narrow tube. If one of the vessels is now maintained at 300 K and the other at 400 K, what will be the pressure in the vessels ?
Sol. Let the initial pressure, volume and temperature in each vessel be p0(=105 kPa), V0 and T0(=300 K). Let the number of moles is each vessel be n. When the first vessel is maintained at temperature T0 and the other is maintained at T’ = 400 K, the pressure change. Let the common pressure becomes p’ and the number of moles in the two vessels become n1 and n2. We have
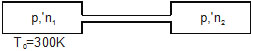
p0V0 = nRT0 ....(i)
p’V0 = n1RT0 ....(ii)
p’V0 = n2RT’ ....(iii)
and n1 + n2 = 2n ....(iv)
Putting n, n1 and n2 from (i), (ii) and (iii) in (iv),
p'VB/RT0 + p'V0/RT' = 2(p0V0/RT0)
or, p(T'+T0/T0T') = 2p0/T0
or, p’ = 2p0T'/T'+T0 = 2 x 105kPa x 400k/ 400k+300k = 120 kPa.
8. A vessel contains 14 g of hydrogen and 96 g of oxygen of STP. (a) Find the volume of the vessel. (b) Chemical reaction is induced by passing electric spark in the vessel till one of the gases is consumed. The temperature is brought back to its starting value 273 K. Find the pressure in the vessel.
Sol. (a) The number of moles of hydrogen = 14g / 2g = 7 and the number of moles of oxygen = 96 g / 32 g = 3. The total number of moles in the vessel = 7 + 3 = 10. The pressure is 1 atm = 1.0 × 105 N/m2 and the temperature = 273 K.
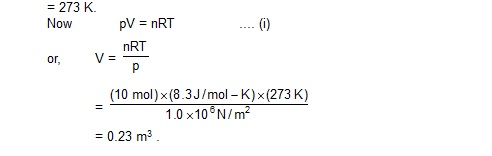
(b) When electric spark is passed, hydrogen reacts with oxygen to form water (H2O). Each gram of hydrogen reacts with eight grams of oxygen. Thus,. 96 g of oxygen will be totally consumed together with 12 g of hydrogen. The gas left in the vessel will be 2 g of hydrogen which is n’ = 1 mole.
Neglecting the volume of the water formed,
p’V = n’RT. ...(ii)
From (i) and (ii),
p'/p = n'/n = 1/10
or, p’ = p × 0.10
= 0.10 atm.
9. A borometer reads 75 cm of mercury. When 2.0 cm3 of air at atmospheric pressure is introduced into the space above the mercury level, the volume of this space becomes 50 cm3. Find the length by which the mercury column descends.
Sol. Let the pressure of the air in the barometer by p. We have,
p × 50 cm3 = (75 cm of mercury) × (2.0 cm3)
or p = 3.0 cm of mercury.
The atmospheric pressure is equal to the pressure due to the mercury column plus the pressure due to the air inside. Thus, the mercury column descends by 3.0 cm.
10. A barometer tube is 1 m long and 2 cm2 in cross-section. Mercury stands to a height of 75 cm in the tube. When a small amount of oxygen is introduced in the space above the mercury level, the leve falls by 5 cm. Calculate the mass of the oxygen intorduced. Room temperature = 27ºC, g = 10 m/s2 and density of mercury = 13600 kg/m3.
Sol. The pressure of oxygen in the space above the mercury level = 5 cm of mercury
= 0.05 m × 13600 kg/m3 × 10 m/s2
= 6800 N/m2.
The volume of oxygen = (2 cm2) × (25 cm + 5 cm)
= 60 cm3 = 6 × 10–5 m3.
The temperature = (273 + 27) K = 300 K.
The amount of oxygen is
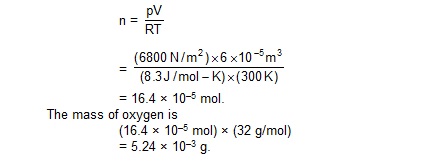
11. Figure shows a vertical cylindrical vessel separated in two parts by a frictionless pistion free to move along the length of the vessel. The length of the cylinder is 90 cm and the piston divides the cylinder in the ratio of 5 : 4. Each of the two parts of the vessel contains o.1 mole of an ideal gas. The temperature of the gas is 300 K is each part. Calculate the mass of the piston.
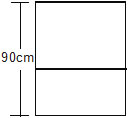
Sol. Let l1 and l2 be the lengths of the upper part and the lower part of the cylinder respectively. Clearly, l1 = 50 cm and l2 = 40 cm. Let the pressure in the upper and lower parts be p1 and p2 respectively. Let the area of cross-section of the cylinder be A. The temperature in both parts is T = 300 K.
Consider the equilibrium of the pistion. The forces acting onthe piston are
(a) its weight mg
(b) p1 A downward, by the upper part of the gas and (c) p2 A upwards, by the lower part of the gas.
Thus, p2 A = p1 A + mg .... (i)
Using pV = nRT for the upper and the lower parts
p1L1A = nRT .....(ii)
and p2L2A = nRT. .....(iii)
Putting p1A and p2A from (ii) and (iii) into (i).
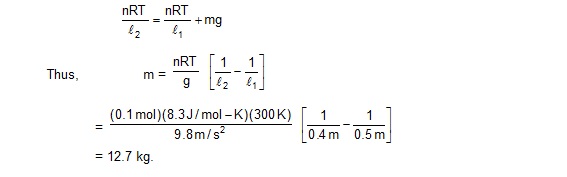
12. Figure shows a cylindrical tube of volume V0 divided in two parts by a frictionless separator. The walls of the tube are adiabatic but the separator is conducting. Ideal gases are filled in the two parts. When the separation is kept in the middle, the pressure are p1 and p2 in the left part and the right part respectively. The separation is slowly slid and is released at a position where it can stay in equilibrium. Find the volume of the two parts.

Sol. As the separation is conducting, the temperatures in the two parts will be the same. Suppose the common temperature is T when the separation is in the middle. Let n1 and n2 be the number of moles of the gas in the left part and the right part respectively. Using ideal gas equation,
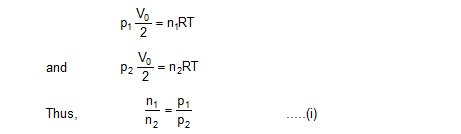
The separator will stay in equilibrium at a position where the pressures on the two sides are equal. Suppose the volume of the left part is V1 and of the right part is V2 in this situation. Let the common pressure be p’. Also, let the common temperature in this situation be T’. Using ideal gas equation,
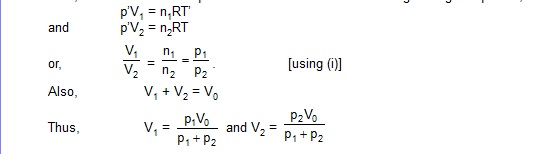
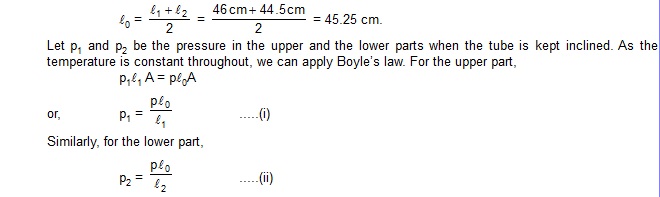
13. A thin tube of uniform cross-section is sealed at both ends. It lies horizontally, the middle 5 cm containing nercury and the parts on its two sides containing air at the same pressure p. When the tube is held at an angle of 60º with the vertical, the length of the air column above and below the mercury pallet are 46 cm and 44.5 cm respectively. Calculate the pressure p in centimetres of mercury. The temperature of the system is kept at 30ºC.
Sol. When the tube is kept inclined to the vertical, the length of the upper part is l1 = 46 cm and that of the lower part is l2 = 44.5 cm. When the tube lies horizontally,the length on each side is
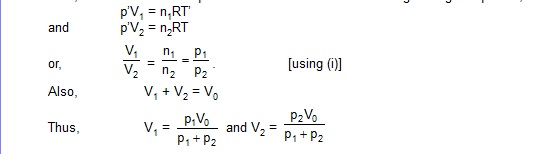
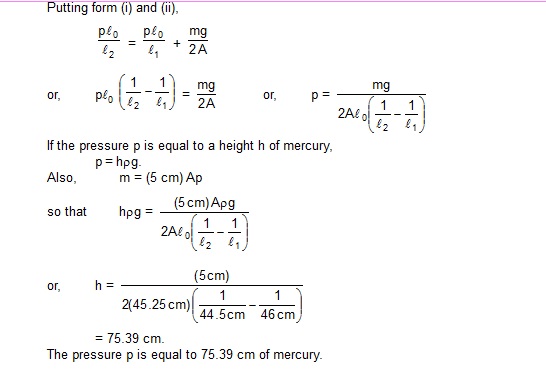
Now consider the equilibrium of the mercury pallet when the tube is kept in inclined position. Let m be the mass of the mercury. The forces along the length of the tube are
(a) p1 A down the tube
(b) p2 A up the tube
and (c) mg cos 60º down the tube.
Thus, p2 = p1 + mg/A cos 60º
14. A ideal monatomic gas is confined in a cylinder by a spring-loaded piston of cross-section 8.0 × 10–3 m2. Initially the gas is at 300 K and occupies a volume of 2.4 × 10–3 m3 and the spring is in its relaxed state (figure). The gas is heated by a small heater until the pistion moves out slowly by 0.1 m. Calculate the final temperature of the gas. The force constant of the spring is 8000 N/m, and the atmospheric pressure is
1.0 × 10 5 N/m2. The cylinder and the piston are thermally insulated. The piston and the spring are masseless and there is no friction between the piston and the cylinder. Neglect any heat-loss through the lead wires of the heater. The piston and the spring are massless and there is no friction between the piston and the cylinder. Neglect any heat-loss through the lead wires of the heater. The heat capacity of the heater coil is neglibible.
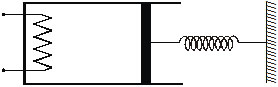
Sol. Initially the spring is in its relaxed state. So, the pressure of the gas equals the atmospheric pressure.
Initial pressure = p1 = 1.0 × 10 5 N/m2.
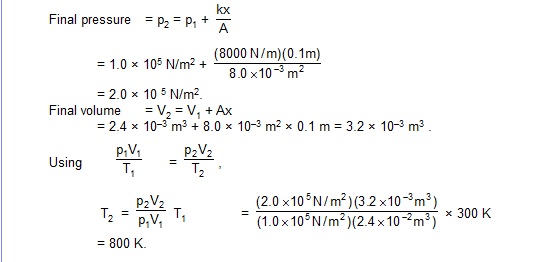
15. Assume that the temperature remains essentially constant in the upper part of the atmosphere. Obtain an expression for the variation in pressure in the upperatmosphere with height. The mean molecular weight of air is M.
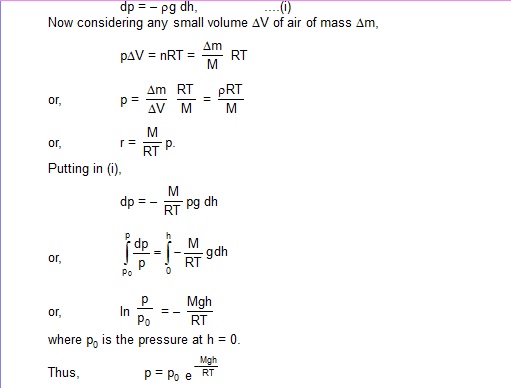
Sol. Suppose the pressure at height h is p and that at h + dh is p + dp. Then
dp = – pg dh, ....(i)
Now considering any small volume DV of air of mass Dm,
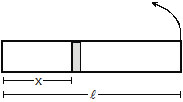
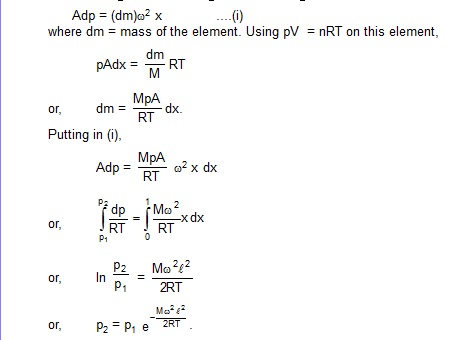
16. A horizontal tube of length l closed at both ends contains an ideal gas of molecules weight M. The tube is rotated at a constant angular velocity w about a vertical axis passing through an end. Assuming the temperature to be uniform and constant, show that
![]()
where p2 and p1 denote the pressure at the free end and the fixed end respectively.
Sol. Consider an element of the gas between the cross-sections at distances x and x + dx from the fixed end (figure). If p be the pressure at x and p + dp at x + dx, the force acting on the element towards the centre is Adp, where A is the cross-sectional area. As this element is going in a circle of radius x,
17. A barometer tube contains a mixture of air and saturated water vapour in the space about the mercury column. It reads 70 cm when the actual atmospheric pressure is 76 cm of mercury. The saturation vapour pressure at room temperature is 1.0 cm of mercury. The tube is now lowered in the reaservoir till the space above the mercury column is reduced to half its original volume. Find the reading of the barometer. Assume that the temperature remains constant.
Sol. The pressure due to the air + vapour is 76 cm – 70 cm = 6 cm of mercury. The vapour is saturated and the pressure due to it is 1 cm of mercury. The pressure due to the air is, therefore, 5 cm of mercury.
As the tube is lowered and the volume above the mercury is decreased, some of the vapour will condense. The remaining vapour will again exert a pressure of 1 cm of mercury. The pressure due to air is doubled as the volume is halved.
Thus, pair = 2 × 5 cm = 10 cm of mercury.
The pressure due to the air + vapour = 10 cm + 1 cm = 11 cm of mercury.
The barometer reading is 76 cm – 11 cm = 65 cm.
18. Find the mass of water vapour per cubic metre of air at temperature 300 K and relative humidily 50%. The saturation vapour pressure at 300 K is 3.6 kPa and the gas constant R = 8.3 J/mol -K.
Sol. At 300 K, the saturation vapour pressure = 3.6 kPa. Considering 1 m3 of volume,
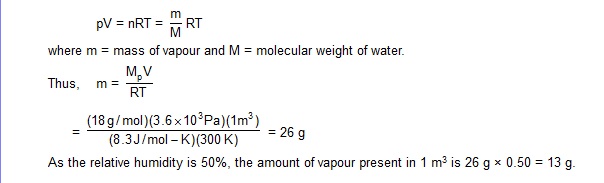
19. The temperature and the relative humidity of air are 20ºC and 80% on a certain day. Find the fraction of the mass of water vapour that will condence if the temperature falls to 5ºC. Saturation vapour pressures at 20ºC and 5ºC are 17.5 mm and 6.5 mm of mercury respectively.
Sol. The relative humidity is
![]()
Thus, the vapour pressure at 20ºC
= 0.8 × 17.5 mm of Hg
= 14 mm of Hg.
Consider a volume V of air. If the vapour pressure is p and the temperature is T, the mass m of the vapour present is given by

When the air is cooled to 5ºC, some vapour condenses and the air gets saturated with the remaining vapour. The vapour pressure at 5ºC is, therefore, 6.5 mm of mercury. The mass of vapour present at 5ºC is, therefore,
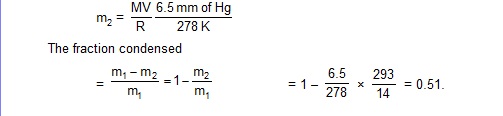
20. A vessel containing wate is put in a dry sealed room of volume 76m3 at a temperature of 15ºC. The saturation vapour pressure of water at 15ºC is 15 mm of mercury. How much water will evaporate before the water is in equilibrium with the vapour?
Sol. Water will be in equilibrium with its vapour when the vapour gets saturated vapour pressure = 15 mm of mercury
= (15 × 10–3 m) (13600 kg/m2) (9.8 m/s2)
= 2000 N/m2.
Using gas law, pV = m/M RT
m = MpV/RT = (18/mol)2000N/m2)(76m3) / (8.3J/mol - K)(288K)
Thus, 1.14 kg of water will evaporate.
21. A jar contains a gas and a few drops of water at absolute temperature T1. The pressure in the jar is 830 mm of mercury. The temperature of the jar is reduced by 1%. The saturation vapour pressures of water at the two temperatures are 30 mm of mercury and 25 mm of mercury. Calculate the new pressure in the jar.
Sol. At temperature T1, the total pressure is 830 mm of mercury. Out of this, 30 mm of mercury is due to the vapour and 800 mm of mercury is due to the gas. As the temperature decreases, the pressure due to the gas decreases according to the gas law. here the volume is constant, so,
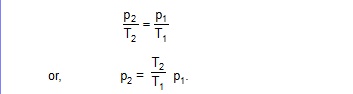 .
.
As T2 is 1 % less than T1
T2 = 0.99 T1
and hence,
p2 = 0.99 p1
0.99 × 800 mm of mercury = 792 mm of mercury.
The vapour is still saturated and hence, its pressure is 25 mm of mercury. The total pressure at the reduced temperature is
p = (792 + 25) mm of mercury.
= 817 mm of mercury.
22. Calculate the mass of 1 litre of moist air at 27ºC when the barometer reads 753.6 mm of mercury and the dew point is 16.1ºC. Saturation vapour pressure of water at = 0.001293 g/cc, density of saturated water vapour at STP = 0.000808 g/cc.
Sol. We have pV = m/M RT
or p = m/V = Mp/RT ... (i)
The dew point is 16.1ºC and the saturation vapour pressure is 13.6 mm of mercury at the dew point. This means that the present vapour pressure is 13.6 mm of mercury.
At this pressure and temperature, the density of vapour will be

Thus, 1 litre of moist air at 27ºC contains 0.0131 g of vapour.
The pressure of dry air at 27ºC is 753.6 mm – 13.6 mm = 740 mm of mercury. The density of air at STP is 0.001293 g/cc. the density at 27ºC is given by equation (i),

= 0.001457 g/cc.
Thus, 1 litre of moist air contains 1.145 g of dry air. The mass of 1 litre of moist air is 1.1457 g + 0.0131 g » 1.159 g.