Everyday you come across many changes in your surroundings out of these changes some are temporary in nature, while some are permanent in nature and hence these changes are broadly categorised into two types
(i) Physical Changes
(ii) Chemical Changes
(a) Physical changes are those changes which are reversible in nature.
(b) These are temparory in nature and also called short duration changes.
(c) These changes are those in which after the accomplishment of the reaction no new substance is formed.
(d) Properties such as shape, size, colour and state are called physical properties any change in these properties of a substance is called a physical change.
(e) For example
(i) Melting of wax (ii) Melting of ice (iii) Evaporation of water
(iv) Formation of dew (v) Sublimation of comphor
(vi) Crystallization of sugar from its solution
(vii) Lightning of a bulb
(a) Those changes in which new substances are formed are called chemical changes, the substances changes their identity and get converted into entirely new substance.
(b) The new substances usually cannot be reformed back in to their original form, simply these are usually irreversible in nature.
(c) These changes are parmanent in nature.
(d) The properties of products are totally different from that of reactants.
(e) For example
(i) Burning of candle
(ii) Burning of fuels (coal, wood, and LPG)
(iii) Digestion of food
(iv) Cooking of food
(v) Formation of curd from milk
(vi) Decomposition of water into H2 and O2 by passing electricity
When magnesium wire or ribbon, burn it starts burning with a dazzling white flame.
(vii) 2Mg + O2 ® 2MgO , MgO + H2O ® Mg(OH)2
(viii) CuSO4 + Fe ® FeSO4 + Cu
Blue Light green
(ix) CH3COOH + NaHCO3 ® CH3COONa + CO2 + H2O
CO2 + Ca(OH)2 ® CaCO3 + H2O
white ppt.
(i) Heat, light or any other radiation (ultraviolet for example) may be given off or absorbed
(ii) Sound production may take place
(iii) A change in smell may takes place
(iv) there may be change in the state
(v) there may be change in the colour
(vi) evolution of gas may takes place
(vii) Change in temperature may be observed.
(viii) Formation of precipitate may be observed.
(i) Burning is a type of chemical change which is always accompanied by the evolution of heat.
(ii) Explosion is also a chemical change because, explosion produces heat, light, sound and certain harmful gases.
(iii) Neutralization of an acid with a base is a type of chemical change which results in the formation of salt and water with the evolution of heat.
Ozone layer (stratosphere) protects us from the harmful ultraviolet radiations from the Sun. By absorbing this radiation it splits up into oxygen (O2) and nascent oxygen (O). We can call this change a chemical change. In this manner ozone protects us from the harmful effects of ultraviolet radiation like skin cancer, global warming, melting of glaciers etc ozone acts as a natural shield against the radiation.
(i) Rusting of iron is also called corrosion of iron
(ii) Corrosion is defined as the gradual transformation of a metal into its combined state because of the reaction with the environment.
(iii) Reaction for rusting
4Fe + 3O2 + 2H2O ® 2Fe2O3.2H2O
(iv) The composition of the rust mainly contains hydrated ferric oxide (Fe2O3.2H2O) with small quantity of ferrous carbonate (FeCO3).
(v) Favourable conditions for rusting of iron are given below:
(a) presence of moisture (b) Presence of weakly acidic environment
(c) presence of oxygen (d) presence of impurity in the iron
(vi) Corrosion is a surface phenomenon and thus, the protection of the surface prevents the corrosion,
(vii) Corrosion can be prevented by applying following methods:
(a) Applying paints, lacquers and enamels on the surface of iron.
(b) By depositing a layer of a metal like chromium or zinc on iron. The process of depositing a layer of zinc on iron is called galvanization. Hence, iron pipes which we use in our homes are galvanized to prevent rusting.
(viii) If the moisture content of the atmosphere is high, the rusting reaction takes place at a faster rate.
(ix) Ships suffer a lot of damage from rusting inspite of being painted, because sea water contains many salts, which makes the process of rust formation faster.
CRYSTALLIZATION
The process of cooling a hot concentrated solution of a substance to obtain crystals is called crystallization. Crystallization is a physical change.
You know that ice, on keeping at open place starts melting and water on boiling starts evaporating. Similarly when vapours are cooled down they come into the liquid state and liquid on further cooling get freezed into solid state (ice).
Ice, water and vapour are the three states of water.
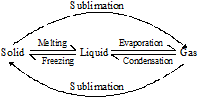
It means on changing temperature states can be changed.
(i) Solid to liquid (Melting or Fusion)
(a) Take a wax candle and burn it, you will observe that wax on burning is converted into liquid state. Normally, it is called melting of wax.
(b) The conversion of a solid substance into liquid state is called ‘Melting or Fusion’
(c) Now look towards the melting wax which is going downwards by the stick of the candle, after some time it will again converted into the solid state.
(d) The conversion of liquid state into solid state, by cooling is called freezing.
(e) When solid substance is heated it gets converted into liquid state the melting of a solid substance takes place at a definite temperature?
(f) “Definite temperature at which a solid substance starts melting by heating is called its melting point”
(ii) Liquid to Solid (Freezing)
(a) “Definite temperature at which a liquid substance (state) starts to convert into the solid substance (state) is called freezing point of the liquid”
(iii) Liquid to Gas (Boiling or Vaporization)
“The conversion of liquid state into the gaseous state on heating is called vaporization or evaporation or boiling”
(c) During boiling temperature remains constant.
(d) The definite temperature at which a liquid boils and changes rapidly into a gas at atmospheric pressure, is called boiling point of the liquid.
(e) The boiling point of water is 100°C and of Acetone is 56°C.
(iv) Gas to Liquid (Condensation)
(a) The process of conversion of gaseous state into the liquid state on cooling, is called condensation.
For example, Formation of dew drops
(v) Solid to gas (Sublimation)
(a) Some substances like Camphor does not go into liquid state it directly enters into the vapour state and then gets converted into the solid state again on cooling.
(b) Some solids directly, goes into the vapour state without passing from the liquid state, on heating and gets converted again into the solid state on cooling, these solid substances are called sublime solid sand this process is called sublimation.
For example, Iodine, Camphor, Napthalene, Ammonium chloride are some sublime solids.
OBJECTIVE QUESTIONS
1. Physical chemical changes are a result of
absorption of :
(A) heat energy only
(B) light energy only
(C) sound energy only
(D) some kind of energy
2. A chemical change involves :
(A) change of state only
(B) change of colour only
(C) change of state as well as composition
(D) None of these
3. A physical change is a/an :
(A) temporary change
(B) permanent change
(C) irreversible change
(D) None of these
4. An example of a physical change is:
(A) magnetisation of iron
(B) burning of wood
(C) photosynthesis by plants
(D) digestion of food
5. An example of a chemical change is :
(A) exposure of a photographic plate
(B) tearing of paper
(C) melting of sulphur
(D) formation of dew
6. An example of an undesirable change is :
(A) melting of snow on mountains
(B) evaporation of water
(C) earthquake
(D) flowering of plants
7. An example of a periodic change is :
(A) swinging of a clock pendulum
(B) landslides during rains
(C) rusting of iron
(D) sneezing
8. During physical and chemical changes :
(A) energy is always absorbed
(B) energy is always released
(C) no energy is absorbed or released
(D) energy is either absorbed or released
9. A change is an alteration in the physical and chemical properties of matter due to the effect of :
(A) light
(B) heat
(C) some kind of energy
(D) pressure
10. Physical changes are :
(A) permanent
(B) irreversible
(C) temporary and irreversible
(D) temparary and can be reversed
SUBJECTIVE QUESTIONS
1. What is change ? Explain giving example.
2. (i) Define a chemical change.
(ii) Give four examples of chemical change
3. Classify the following changes as fast or slow changes :
(i) Germination of seed
(ii) Milk changing to curd
(iii) Burning of cooking gas in a gas stove
4. Define and explain periodic changes.
1. D 2. C 3. A 4. A
5. A 6. C 7. 8. D
9. C 10. D 11. C 12. A
13. A
1. The valency of phosphate ion is
(A) –1 (B) –2 (C) +3 (D) –3
2. The reaction 2Na + Cl2 ® 2NaCl is an example of
(A) Combination reaction (B) Decomposition reaction
(C) Displacement reaction (D) Double decomposition reaction
3. Which of the following reaction involves the combination of two elements ?
(A) CaO + CO2 ® CaCO3 (B) 4Na + O2 ® 2Na2O
(C) SO2 + O2 ® SO3 (D) NH3 + HCL ® NH4C1
4. When lead nitrate is heated, it breaks down into lead monoxide, nitrogen dioxide and oxygen
2Pb(NO3)2 ® 2PbO + 4NO2 + O2
This reaction is an example of
(A) Combination reaction (B) Decomposition reaction
(C) Double decomposition reaction (D) Displacement reaction
5. Which of the following is a displacement reaction ?
(A) NaOH + HNO3 ® NaNO3 + H2O (B) Cu + 2AgNO3 ® Cu(NO3)2 + 2Ag
(C) 2Hg + O2 ® 2HgO (D) FeCl3 + 3NaOH ® 2NaCl + Fe(OH)3
6. Which of the following reaction will not take place ?
(A) Zn + FeSO4 ® ZnSO4 + Fe (B) 2KI + CI2 ® 2KCI + I2
(C) Zn + MgSO4 ® ZnSO4 + Mg (D) Mg + CuSO4 ® MgSO4 + Cu
7. The reaction in which two compunds exchange their ions to form two new compounds is called
(A) A displacement reaction (B) A decomposition reaction
(C) An isomerization (D) A metathesis reaction
8. When hydrogen sulhide gas is passed through a blue soluation of copper sulphate, a black precipitate of copper sulphuric acid to formed remains in the solution. The reaction is an example of
(A) A combination reaction (B) A displacement reaction
(C) A decomposition reaction (D) A double decomposition reaction
9. When the gases sulphur dioxide and hydrogen sulphide mix in the presence of water, the reaction
SO2 + 2H2S ® 2H2O + 3S occurs.
Here hydrogen sulphide is acting as
(A) An oxidising agent (B) A reducing agent
(C) A dehydrating agent (D) A catalyst
10. In the reaction : 2FeCI2 + CI2 ® 2FeCI3 Chlorine may be regarded as
(A) An oxidising agent (B) A reducing agent
(C) A Catalyst (D) Providing an inert medium
11. In the following equations : Na2CO3 + cHCI ® 2NaCI + CO2 + H2O the vale of c is
(A) 1 (B) 2 (C) 3 (D) 4
12. The euations : Cu + X HNO3 ® Cu(NO3) + Y NO2 + 2H2O3
the values of X & Y are
(A) 3 and 1 (B) 8 and 6 (C) 4 and 2 (D) 7 and 1 respectively
13. In the equation : NaOH + HNO3 ® NaNO3 + H2O2 nitric acid is acting as
(A) An oxidising agent (B) An acid
(C) A nitrating agent (D) A dehydrating agent.
14. Which of the following is not a balanced equation ?
(A) Ca(HO)2 + CO2 ¾® CaCO3 + H2 O (B) Fe + CuSO4 ¾® FeSO4 + Cu
(C) KCIO4 KCI + 2O2 (D) Cu +2HNO3 ¾® Cu(NO3)2 + 2NO3 + H2O
15. Combustion of fuel is
(A) Displacement reaction (B) Dobule displacement reaction
(C) Oxidation Reaction (D) isomerisation reaction
16. BaCI2 + H2SO4 ® BaSO4 + 2HCI is
(A) Combination reaction (B) Decomposition reaction
(C) Displacement reaction (D) Double displacement reaction
17. Zinc can displace copper from copper sulphate solution because zinc is
(A) More reactive than copper (B) less reactive than copper
(C) more stable than copper (D) less stable than copper
18. When Magnesium is burnt in air, a white ash remain as left over. What is this ?
(A) MgO2 (B) MgO (C) Mg (D) Mg3O
19. Carbon dioxide reacts with carbon to form carbon monoxide. This is an example of a
(A) Displacement reaction (B) Double displacement reaction
(C) Combination reaction (D) Decomposition reaction
20. CaCO3 CaO + CO is known as
(A) Electrolysis (B) Thermal decomposition
(C) Displacement (D) Combination
21. Which of the following is a decomposition reaction ?
(A) 2KBr + CI2 ® 2KCI + Br2 (B) 2Na + H2O ® 2NaOH + H2
(C) NH4CNO ® NH2CONH2 (D) ZnCO3 ® ZnO + CO2
22. The formula of silver phosphate is
(A) AgPO4 (B) Ag3PO4 (C) Ag2(PO4)3 (D) Ag2PO4
23. Which of the following is example of combination reaction ?
(A) H2 + CI2 ® 2HCI (B) n-Hexance neo hexance
(C) Zn + H2SO4 ® ZnSO4 + H2 (D) N2O4 ® 2NO2
24. Which of the following reactions is not a decomposition reaction ?
(A) Starch is converted to glucose in our body
(B) Proteins are digested to amino acids
(C) Magnesium ribbon is burnt in air
(D) Electricity is passed through water
25. The reaction between acid and base to form salt and water is an example of
(A) Decomposition reaction (B) Combination reaction
(C) Displacement reaction (D) Double displacement
26. Which of the following statement is incorrect ?
(A) Metal like Cu, Ag, Au cannot displace H from acids
(B) In reactivity series metals are arranged in order of increasing reactivity
(C) Silver cannot displace Cu from Cu(NO3)2
(D) Zinc displaces Cu from CuSO4
27. In the balanced reaction : aFe2O3+ bH2 ® cFe + dH2O
a, b, c and d, respectively, are
(A) 1123 (B) 1111 (C) 1323 (D) 1223
28. Which of the following reaction are exothermic in nature ?
(A) Combustion of carbon (B) Bond breaking
(C) Bond formation (D) Both (A) & (C)
29. Which of the following chages in not a physical change ?
(A) Glowing of felament in bulb (B) Combustion
(C) Boiling of water (D) Sublimation
30. The substance that looses electrons is called as
(A) Oxidizing agent (B) Reducing agent
(C) Catalyst (D) None of these
31. in the reaction Mg + CI2 ® Mg CI2
(A) Magnesium is oxidized and CI2 is reduced
(B) Magnesium is reduced and CI3 is oxidized
(C) Magnesium gains 2 electrons
(D) Clorine losses 1 electron
32. When Zn chages Zn+2 it
(A) losses 2 electrons (B) Looses 1 electrons
(C) gains 1 electrons (D) Gains 2 electrons
33. The process of reduction involves
(A) Removal of hydrogen (B) Gain of electrons
(C) addition of oxygen (D) loss of electrons
34. In the reaction 3MnO2 + 4AI ® 3Mn + 2AI2O3, the oxidizing agent ss
(A) MnO2 (B) AI (C) AI2O3 (D) Mn
35. In the reaction H2S + CI2 ® 2HCI + S, the oxidizing agent is
(A) H2S (B) CI2 (C) HCI (D) S
36. Which of the following statement is correct ?
(A) Oxidation involves gain of electrons
(B) Substance which is reduced in reducing agent
(C) Exothermic process involves absorption of heat
(D) Oxidation involves loss of electrons
37. Select redox reaction from the following
(A) Zn + CuSO4 ® ZnSO4 + Cu (B) CaO + 2HCI ® CaCI3 + H2O
(C) NaOH + 2HCI ® NaCI2 + H2O (D) CaCO3 ® CaO + CO2
38. In the reaction : 2FeCI3 + SnCI2 ® 2FeCI2 + SnCI4
(A) Fe+3 reduced to Fe+2 (B) Sn+2 is reduced to Sn+4
(C) Sn+2s oxidized to Sn (D) Fe+3 gains two electrons
39. Which of the following reactions in not correct
(A) 2AgNO + Cu ® Cu(NO3)2 + 2Ag (B) CI2 + 2KI ® 2KCI + I2
(C) FeSO4 + Cu ® CuSO4 + Fe (D) CuSO4 + Zn ® ZnSO4 + Cu
40. In the reaction Zn + FeSO4 + ZnSO4 + Fe
(A) Zn gets oxidize (B) Fe gets oxidized
(C) Zn is oxidized in agent (D) Zn and Fe both get oxidized
41. Consider the reaction
CuSO4 + Fe ® FeSO4 + Cu
FeSO4 + Zn ® Zn SO4 + Fe
(A) Zn is most reactive, Fe is least reactive
(B) Fe is most reative and Cu is least reactive
(C) Zn is mos reactive and Cu is least reactive
(D) Cu is most reactions, Fe is least reactive
42. Which of the following reaction is not endothermic in nature ?
(A) Breaking of bonds (B) Digestion of food
(C) Combustion of Carbon (D) Evaporation of water
43. Chosse the incorrect statement.
(A) Physical chage is reversible (B) Physcial change results information of new substances
(C) Chemical change is permanent (D) Physical chage is accompained by energy chage
44. Which of following is fast reaction ?
(A) Reaction between H2 and O2 to from H2O
(B) Reaction between acid and base to form salt and water
(C) Hydrolysis of ester
(D) Hydrolysis of suger to glucose
ANSWER KEY
1. B 2. A 3. B 4. B 5. B 6. C 7. D 8. D 9. B 10. A 11. B 12. C 13. B 14. D 15.
C 16. D 17. A 18. B 19. B 20. B 21. D 22. B 23. A 24. C 25. D 26. B 27. C 28. D
29. B 30. B 31. A 32. A 33. B 34. A 35. B 36. D 37. A 38. A 39. C 40. A 41. C 42. C 43. B 44. B
BOARD PROBLEMS
Section-A
• Fill in the blanks
1. When carbon dioxide is passed through lime water, it turns milky due to the formation of __________.
2. The chemical name of baking soda is ______________.
3. Two methods by which rusting of iron can be prevented are ____________ and ______________.
4. Changes in which only ____________properties of a substance change are called physical changes.
5. Changes in which new substances are formed are called ______________changes.
6. Boiling of water is a ______________change.
7. Conversion of solid into ______________is called melting.
8. The mass of a substance does alter in a ______________change.
9. Sublimation of solid is a______________change.
Section-B
• True or False
1. Ozone acts as a natural shield against U.V. radiation.
2. Rust is hydrated ferric oxide
3. Lime water turns milky by passing CO2 due to the formation of white ppt. of CaCO3.
4. The colour of CuSO4 solution is green and FeSO4 solution is blue, when the reaction takes place between CuSO4 and Fe.
5. Cutting of paper is a physical change
6. Cutting a log of wood into pieces is a chemical change?
7. Iron pipes coated with zinc do not get rusted easily
8. Iron and rust are the some substances
9. Condensation of steam is not a chemical change.
ANSWERS
Section-A
1. CaCO3 2. Sodium bicarbonate 3. painting, galvanization
4. physical 5. chemical 6. physical
7. Liquid 8. chemical 9. physical
Section-B
1. [T] 2. [T] 3. [T] 4. [F] 5. [T] 6. [F] 7. [T] 8. [F] 9. [T]
NTSE/STSE PROBLEMS
Section-A
• Multiple Choice Questions (NTSE competition Based)
1. Chemical changes are
(A) Temporary, reversible and a new substance is produced
(B) Always accompanied by exchanges of light
(C) Permanent, irreversible and a new substance in produced
(D) Never accompanied by exchange of light and heat energy
2. Which of the following is a physical change?
(A) Solubility in water
(B) Formation of curd
(C) Aerial oxidation
(D) Reaction with water
3. The sign used ti indicate a reversible reaction
(A) ® (B) @
(C) ¬ (D)
4. Breaking of lead bromide into lead and bromine is an example of
(A) Decomposition (B) Synthesis
(C) Displacement (D) Neutralization
5. A white solid which is yellow when hot but changes to white again on cooling is
(A) PbO (B) CaO
(C) Ag2O (D) ZnO
6. The products of burning candle are
(A) ash and water vapour
(B) CO2 and water vapour
(C) wax and water vapours
(D) Only metal wax
7. The main cause by rancidity in foods is
(A) Bacteria
(B) Proteins
(C) Antioxidants
(D) Oxidation of the fatty acid molecules
8. The formula of rust is
(A) CuO (B) Al2O3
(C) Fe2O3.xH2O (D) Fe2O3
9. Changing of gas into liquid is called?
(A) Melting (B) Boiling
(C) Condensation (D) Evaporation
10. Shows the characteristics of sublimation
(A) Ice (B) Sugar
(C) Nausadar (D) Alum
11. The gas you use in kitchen is called liquefied petroleum gas (LPG). In the cylinder, it exists as a liquid. When it comes out of the cylinder, it becomes a gas (process A), then it burns (process B). Choose the correct statement -
(a) Process (a) is a chemical change
(b) Process (b) is a chemical change
(c) Both processes (a) and (b) are chemical changes
(d) None of these processes is a chemical change
12. Anaerobic bacteria digest animal waste and produce biogas (change A). The biogas is burnt as a fuel (change B). Choose the correct statement -
(a) Process (a) is a chemical change
(b) Process (b) is a chemical change
(c) Both processes (a) and (b) are chemical changes
(d) None of these changes is a chemical change
13. A chemical reaction, in which heat is evolved is called -
(a) endothermic reaction
(b) exothermic reaction
(c) neutralisation reaction
(d) displacement reaction
14. Slow eating away of iron articles in the presence of moist air is called -
(a) galvanisation
(b) crystallisation
(c) rusting
(d) neutralisation
15. Sun rises in the east and sets in the west, this repeated change in called -
(a) irreversible change
(b) periodic change
(c) physical change
16. When food eaten by us is digested it undergoes ________ change
(a) periodic (b) reversible
(c) irreversible (d) physical
17. Melting of wax is a _______ change, while burning of candle is _____ change
(a) irreversible, reversible (b) reversible, irreversible
(c) physical, reversible
(d) chemical, irreversible
18. Select the reversible changes from the following and choose the answer from the option given below -
(i) melting of wax
(ii) freezing of water
(iii) formation of curd from milk
(a) (i) & (ii)
(b) (i), (ii) & (iv)
(c) (iii) only
(d) All of the above
19. Fraction of ship’s iron has to be replaced every year because -
(a) it prevents rusting of iron (b) its color fades away
(c) ship can move properly in water only after replacement
(d) all the above
20. ___________ is a physical change where surface molecules of a liquid escape -
(a) Evaporation
(b) Condensation
(c) Foaming
(d) None of these
21. Which one of the following is a physical change?
(a) Digestion of food
(b) Boiling of an egg
(c) Making of cup of tea (d) None of these
22. Photosynthesis is a _________
(a) physical change
(b) chemical change
(c) both
(d) None of these
23. The process of obtaining pure crystals of copper sulphate from copper sulphate solution is known as -
(a) crystallisation
(b) galvanisation
(c) rusting
(d) None of these
24. A ________ speeds up a chemical reaction
(a) reactant
(b) product
(c) catalyst
(d) None of these
25. Coating iron with a thin layer of zinc, to prevent rusting is -
(a) galvanization
(b) anodizing
(c) crystallization
(d) None of these
26. Examples of physical properties are -
(a) corrosiveness and strength
(b) flammability and ability to conduct electricity
(c) melting point and solubility (d) None of these
27. The gas in the atmosphere which acts as a natural shiel against ultra violet radiation is -
(a) ozone (b) sulphur dioxide
(c) oxygen (d) None of these
28. Why rusting of iron is faster in coastal areas than in deserts?
(a) Because air has more moisture in coastal areas than in desert areas (b) Because air has less moisture in coastal areas than in desert areas
(c) None of these (d) Both (a) and (b)
29. On a hot summer day ice-cream melts faster than in winters. This process of melting is -
(a) chemical change
(b) physical change
(c) periodic change
(d) irreversible change
30. During rusting, the layer deposited on the surface of iron is -
(a) FeO
(b) Fe2O3
(c) Fe2O3.xH2O
(d) None of these
Section-B
• One Word
1. NH4Cl is a type of?
2. Melting is also called as
3. Changing from solid to gas and gas to solid without passing from liquid state is called
4. What is the SI unit of measuring temperature
5. What is the freezing point of water and melting point of ice?
6. Conversion of liquid into solid is called
ANSWERS
1. (C) 2. (A)
3. (D) 4. (A)
5. (A) 6. (B)
7. (D) 8. (C)
9. (C) 10. (C)