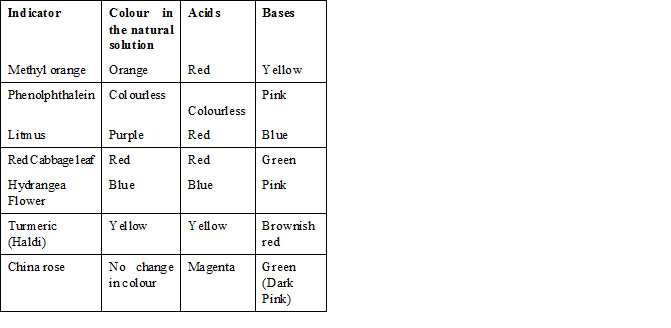
A. Indicators showing different colours in acidic and basic medium
a. Litmus solution as indicator is a purple coloured dye extracted from the lichen plant. It is the most commonly used indicator
in the science laboratory. In the neutral solution, it has purple colour. In the acidic solution, it turns red whereas in the basic solution it turns blue.
There are two types of litmus solution Blue and Red litmus solution. Red litmus solution is obtained by acidifying the purple litmus extract
whereas blue litmus solution is obtained by making the purple litmus extract alkaline.
To test whether the given sample is acidic or basic, take few drops of distilled water in a test tube and two drops of blue litmus solution.
Add few drops of sample substance that is to be tested. If the blue litmus solution changes into red colour, the substance is acidic.
For instance, lemon juice, vinegar, orange juice, juice of unripe mangoes, tamarind all turn blue litmus solution to red. Thus, they are all acidic substances.
We may repeat the above experiment with red litmus solution. Those substances, which turn red litmus solution into blue colour, are bases.
For instance, cucumber, washing soda solution, baking soda solution, bitter gourd etc. turn red litmus solution into blue. Thus, they are bases.
b. Synthetic indicators such as Phenolphthalein and Methyl orange
B. Indicators giving different odours in acidic and basic medium. (Olfactory indicators)
C. Natural Indicators
Turmeric (Haldi), red cabbage, China rose peals are the natural indicators.
An acid (from the Latin acidus meaning sour) is traditionally considered any chemical compound that, when dissolved in water,
gives a solution with a hydrogen ion activity greater than in pure water. Chemicals or substances having the property of an acid are said to be acidic.
Acids are sour in taste. Acids are of organic and inorganic nature. Acids found in plants and animals are organic in nature.
Organic acids are weak whether inorganic acids are strong.
I Organic Acids
II Inorganic acids are called mineral acids. They are prepared by dissolving mineral oxides in water.
Sulphur dioxide dissolves in water to form sulphurous or sulphuric acid. Carbon dioxide dissolves in
water to form carbonic acid. Hydrogen chloride dissolves in water to form hydrochloric acid etc.
Strong bases, like strong acids, attack living tissue and cause serious burns. They react differently to skin than acids do,
so while strong acids are corrosive, we say that strong bases are caustic (corrosive). Bases may also be weak bases such as ammonia,
which is used for cleaning. Arrhenius bases are water-soluble. An alkali is a special example of a base, where in an aqueous environment;
hydroxide ions (also viewed as OH–) are donated. Bases, which dissolve in water, are called alkalis. Bases are alkalis but not all alkalis are bases.
The notion of a base as a concept in chemistry was first introduced by the French chemist Guillaume Francois Rouelle in 1754. He noted that acids,
which in those days were mostly volatile liquids (like acetic acid), turned into solid salts only when combined with specific substances.
These substances form a concrete base for the salt and hence the name.
Some general properties of bases include:
SALT
A salt, in chemistry, is defined as the product formed from the neutralization reaction of acids and bases.
There are several varieties of salts. Salts that produce hydroxide ions when dissolved in water are basic salts
and salts that produce hydronium ions in water are acid salts. Neutral salts are those that are neither acid nor basic salts.
When salts are dissolved in water, they are called electrolytes, and are able to conduct electricity, a property that is shared
with molten salts. Sodium Chloride (NaCl), Magnesium Chloride (MgCl2), Calcium Carbonate (CaCO3), Calcium Sulphate (CaSO4) etc.
are some examples of salt. Not all salt are edible. Salts can be poisonous to the body as well. Not all salts are salty.
Salt that we add to our food is Sodium Chloride (NaCl).
Did you Know
1. Sulphuric acid is called the king of chemicals because of its wide spread use.
2. Bases tat can dissolve in water are called alkali. Sodium hydroxide(NaOH) and potassium hydroxide(KOH) are bases that can readily dissolve in water.
3. Acid rain is formed when pollutants, sulphur dioxide and nitrogen dioxide (NO2) which are released into the atmosphere by burning of fossil fuels.
4. Saliva in the mouth contains mucus, which lubricates food and case its passage into the food pipe is basic in nature.
5. Blood when healthy is also basic in nature.
6. NaOH and KOH are called caustic alkalis’ as they cause skin burns.
7. Sodium iodede contains iodine which is a supplement to common salt(sodium chloride) that prevents the disease.
1. All acids have a sour taste. They turn blue litmus red.
2. Bases are bitter in taste and produce a soapy feeling. They turn red litmus blue.
3. Indicators are substance which help us to identify acids and bases.
4. A salt is produced when an acid is neutralized by a base.
5. Substance that are neither acidic nor basic are called neutral.
6. A reaction in which an acid reacts with a base to form salt and water is called neutralisation reaction.
(A) Answer the following in not more than 20 words.
Q.1 Classify the following substances into acidic and basic substances.
Tomato juice, soap solution, toothpaste, lemon juice, vinegar
Q.2 Name three mineral acids and give their formulae.
Q.3 Define acids
Q.4 Define bases
Q.5 What are soluble bases called ? Give two examples.
Q.6 Define neutral substances and indicators.
(B) Answer the following in not more than 40 words.
Q.1 Name an acidic gas which is discharged into the atmosphere on the
burning of fuels like coal and natural gas. How is this gas formed ?
Q.2 What are the general properties of basic substances ?
(C) Answer the following in not more than 100 words.
Q.1 Write the properties of an acid
Q.2 Describe an activity to show the effect of an acid on carbonates and hydrogencarbonates
Q.3 What is acid rain ? How is it formed ? Mention three bad effects of acid rain.
Q.4 Write a note on the uses of bases.
Q.5 Why factory waste is neutralised before disposing it into the water bodies?
(D) Complete the following.
Q.1 The sour things we eat contain…..
Q.2 Ammonium hydroxide is an…..
Q.3 An acid is……by a base
Q.4 An antacid generally contains……
Q.5 Acid + base --------- +------------
(A) Choose the correct option.
Q.1 Acids are formed when
(A) metals combine with oxygen
(B) oxides of nonmetals dissolve in water
(C) metals react with water
(D) bases dissolve in water
Q.2 Hydrochloric acid can be neutralised by
(A) nitric acid
(B) sulphuric acid
(C) citric acid
(D) sodium hydroxide
Q.3 A soap solution is
(A) acidic
(B) alkaline
(C) neutral
(D) None
Q.4 In a neutralisation reaction, an acid reacts with a base to give
(A) another acid
(B) another base
(C) another acid and another base
(D) a salt and water
(B) Match the columns A and B
1. A B
(a) Hydrochloride acid (i) In storage batteries
(b) Ascorbic acid (ii) Found in yoghurt
(c) Sulphuric acid (iii) In making vinegar
(d) Lactic acid (iv) As bathroom acid
(e) Acetic acid (v) Vitamin C
2. A B
(a) Sodium iodate (i) A food preservative
(b) Calcium sulphate (ii) Used as a fertiliser
(c) Bleaching powder (iii) Present in plaster of Paris
(d) Ammonium sulphate (iv) A disinfectant
(e) Sodium benzoate (v) A supplement to common salt
(C) Tick the correct box.
Q.1 Are most salts neutral ?
Q.2 Are soluble bases called alkalis ?
Q.3 Calcium carbonate when heated gives calcium oxide, which is a base. Will the same base be formed when calcium chloride is heated ?
Q.4 Lemon juice gives carbon dioxide with baking soda. Will it give carbon dioxide with marble too ?
Q.5 Carbon when burnt in air gives an acidic gas. Does sulphur when burnt in air give and an acidic gas ?
ANSWER KEY
(A) Choose the correct option.
1. C 2. D 3. B 4. D
(B) Match columns.
1. (a) – (iv) (b) – (v) (c) – (i)
(d) – (ii) (e) – (iii)
2. (a) – (v) (b) – (iii) (c) – (iv)
(d) – (ii) (e) – (i)
(C) Tick the correct box.
1. No 2. Yes 3. No 4. Yes 5. Yes
CHOOSE CORRECT OPTION
1. Which of the following is a strong acid ?
(A) Lactic acid (B) Ascorbic acid (C) Sulphuric acid (D) Formic acid
2. Which of the following is a strong base ?
(A) Ammonium hydroxide (B) Sodium hydroxide (C) Magnesium hydroxide (D) Copper hydroxide
3. Which of the following compounds is an acid?
(A) Na2O (B) Ca(OH)2 (C) CuO (D) HNO3
4. Which of the following is not a base?
(A) KOH (B) ZnO (C) Al(OH)3 (D) NaCl
5. Which of the following is a strong acid?
(A) H2CO3 (B) CHCOOH (C) HCl (D) HCOOH
6. Which of the following is a dibasic acid?
(A) HCI (B) H3PO4 (C) HNO3 (D) H2SO4
7. Potash alum is a
(A) simple salt (B) complex salt (C) acid salt (D) double salt
8. Acetic acid is a weak acid because
(A) its aqueous solution is acidic (B) it is highly ionized
(C) it is weakly ionized (D) it contains –COON group
9. Dolomite is
(A) an acid salt (B) a mixed salt (C) a normal salt (D) a double salt
10. The reaction, Pb(OH)2 + HNO3 ---> Pb(OH)NO3 + H2O shows that Pb(OH)NO3 is :-
(A) an acid salt (B) a basic salt (C) a base (D) an acid
11. Partial neutralization of a polybasic acid gives
(A) acid salt (B) a basic salt (C) normal salt (D) double salt
12. Strongst salt amongst the following is
(A) NaCl (B) CaCl2 (C) BaSO4 (D) LiCl
13. Which of the following can form more than one acid salt?
(A) CH3COOH (B) H3PO4 (C) CH3CH2COOH (D) ZnO
14. Which of the following is not a base?
(A) KOH (B) Ca(OH)2 (C) K2SO4 (D) ZnO
15. A solution turns blue litmus red. The pH of the solution is probably
(A) 8 (B) 10 (C) 12 (D) 6
16. The type of medicine used to treat indigestion is
(A) antihistamic (B) sulpha drug (C) antacid (D) antibiotic
17. Which one of the following types of medicines is used for treating indigestion ?
(A) Antibiotic (B) Analgesic (C) Antacid (D) Antiseptic
18. Which of the following is an organic acid ?
(A) Hydrochloric acid (B) Sulphuric acid (C) Citric acid (D) Nitric acid
19. Which of the following acids is not used in food stuff ?
(A) Ascorbic acid (B) Hydrochloric acid (C) Tartaric acid (D) Citric acid
20. The acid present in tea is
(A) tannic (B) lactic (C) tartaric (D) citric
21. Ascorbic acid is present in -
(A) milk (B) tea (C) ants (D) lemon juice
22. Acid reacts with metal to form -
(A) salt and CO2 (B) salt and water (C) salt and O2 (D) salt and H2
23. NaHCO3 + A ---> NaCI +CO2 + H2O
What is 'A' in above equation ?
(A) HNO3 (B) HCl (C) Cl2 (D) H2SO4
24. Na2O + 2HCI ---> A + B
What is 'A' and 'B' in above equation ?
(A) NaOH + H2 (B) 2NaCI + H2O (C) NaOH + Cl2 (D) 2NaCl + O2
25. Sulphuric acid is formed when ___________ reacts with water
(A) CO2 (B) P2O5 (C) SO3 (D) O2
26. The acid used in the making of vinegar is
(A) formic acid (B) acetic acid (C) sulphuric acid (D) nitric acid
27. Common name of H2SO4 is -
(A) oil of vitriol (B) muriatic acid (C) blue vitriol (D) green vitriol
28. CuO + (X) ---> CuSO4 + H2O. Here (X) is
(A) CuSO4 (B) HCI (C) H2SO4 (D) HNO3
29. Which is strongest acid among the following ?
(A) HClO4 (B) H2SO4 (C) HCI (D) HBr
30. Arrhenius acid gives -
(A) H+ in water (B) OH– in water (C) both (D) none
31. Which of the following is the weakest base ?
(A) NaOH (B) NH4OH (C) KOH (D) Ca(OH)2
32. Reaction of an acid with a base is known as -
(A) decomposition (B) combination (C) redox reaction (D) neutralization
33. Lime water is -
(A) dilute solution of Ca(OH)2 (B) Mg(OH)2 solution
(C) NaOH solution (D) KOH solution
34. When CO2 is passed through lime water, it turns milky. The milkiness in due to -
(A) CaCO3 (B) Ca(OH)2 (C) H2O (D) CO2
35. Caustic soda is the common name for
(A) Mg(OH)2 (B) KOH (C) Ca(OH)2 (D) NaOH
36. Antacids contain -
(A) weak base (B) weak acid. (C) strong base (D) strong acid
37. Calcium hydroxide (slaked lime) is used in
(A) plastics and dyes (B) fertilizers (C) antacids (D) white washing
38. 2NaOH + MgSO4 ---> ?
(A) MgO + Na2SO4 (B) Mg(OH)2 + Na2SO4 (C) Mg(OH)2 + Na2O (D) MgO + Na2O
39. Aqueous solution of base turn the red litmus into -
(A) green (B) blue (C) yellow (D) black
40. Aqueous solution of Ammonia is -
(A) acidic (B) basic (C) neutral (D) none
41. pH of alkaline solution is -4
(A) pH > 7 (B) pH < 7 (C) pH = 7 (D) pH < 4
42. Bases are the substance which on dissolving in water give
(A) H+ ions (B) OH– ions (C) Ca++ ions (D) Na+ ions
43. Soda-lime is a mixture of
(A) NaOH + CaO (B) Ca(OH)2 +N2O (C) CaO + Ca(OH)2 (D) all are correct
44. Which is strongest base among the following ?
(A) NaOH (B) Mg(OH)2 (C) Fe(OH)3 (D) Be(OH)2
45. Aqueous solution of Na2O will be -
(A) acidic (B) basic (C) neutral (D) none
46. Many salts absorb water from the atmosphere. This property is called
(A) hydration (B) dehydration (C) efflorescence (D) deliquescence
47. A certain metal has an insoluble chloride and an insoluble sulphate. The metal could be
(A) copper (B) sodium (C) potassium (D) calcium
48. Formula of rock salt is
(A) CaCl2 (B) KCI (C) NaCI (D) MgCl2
49. Phenolphthalein turns _______ in acidic solution.
(A) colourless (B) pink (C) red (D) green
50. Which of the following is an acidic salt ?
(A) ZnSO4 (B) CH3COONa (C) NaCI (D) Na2SO4
51. The metal which can displace zinc from its salt solution is -
(A) Mg (B) Fe (C) Pb (D) Cu
52. In bases, methyl orange
(A) turns green (B) turns black (C) turns red
(D) shows no change in colour.
53. Indicators are
(A) weak electrolytes (B) strong electrolytes (C) neutral (D) none of these
54. Which is an acidic indicator ?
(A) Phenolphthalein (B) Methyl orange (C) (A) & (B) both (D) none
55. The formula of alum is -
(A) K2SO4.Al2(SO4)3.24H2O (B) K2SO4.Al2(SO4)3.12H2O
(C) (A) & (B) both (D) Na2CO3.10H2O
56. Aqueous solution of FeCl3 will be
(A) acidic (B) basic (C) neutral (D) none
57. The acid used in making of vinegar is -
(A) Formic acid (B) Acetic acid (C) Sulphuric acid (D) Nitric acid
58. Common name of H2SO4 is -
(A) Oil of vitriol (B) Muriatic acid (C) Blue vitriol (D) Green vitriol
59. CuO + (X) ---> CuSO, + H2O. Here (X) is
(A) CuSO4 (B) HCI (C) H2SO4 (D) HNO3
60. Which of the following is the weakest base ?
(A) NaOH (B) NH4OH (C) KOH (D) Ca(OH)2
61. Reaction of an acid with a base is known as
(A) decomposition (B) combination (C) redox reaction (D) neutralization
62. When CO2 is passed through lime water, it turns milky. The milkiness is due to the formation of -
(A) CaCO3 (B) Ca(OH)2 (C) H2O (D) CO2
63. Caustic soda is the common name -for -
(A) Mg(OH)2 (B) KOH (C) Ca(OH)2 (D) NaOH
64. Antacids contain -
(A) Weak base (B) Weak acid (C) Strong base (D) Strong acid
65. Calcium hydroxide (slaked, lime) is used in -
(A) Plastics and dyes (B) Fertilizers (C) Antacids (D) White washing
66. Acids gives :-
(A) H+ in water (B) OH– in water (C) Both (A) & (B) (D) None of these
67. H2CO3 is a -
(A) strong acid (B) weak acid (C) strong base (D) weak base
68. A solution turns red litmus blue. Its pH is likely to be
(A) 2 (B) 4 (C) 7 (D) 10
69. If pH of any solution is equal to zero then solution will be -
(A) acidic (B) basic (C) neutral (D) none of these
70. Methyl orange is
(A) an acidic indicator (B) a basic indicator (C) a neutral indicator (D) none of these
71. pH of Blood is
(A) 6.4 (B) 7.4 (C) 4.7 (D) 6.4
72. If pH of solution is 13, means that it is
(A) weakly acidic (B) weakly basic (C) strongly acidic (D) strongly basic
73. Which is a base and not an alkali ?
(A) NaOH (B) KOH (C) Fe(OH)3 (D) none is true
74. Energy released in neutralisation reaction which occurs between strong acid and strong base is -
(A) 57.8 kJ (B) 57.1 kJ (C) 57.9 kJ (D) 56.1 kJ
75. A solution has pH 2. It contains
(A) CH3COOH (B) H2CO3 (C) HNO3 (D) H2C2O4
76. A solution has pH 9. On dilution the pH value
(A) decreases (B) increases (C) remain same (D) none of these
77. A salt derived from strong acid and weak base will dissolve in water to give a solution which is
(A) acidic (B) basic (C) neutral (D) none of these
78. Materials used in the manufacture of bleaching powder are
(A) lime stone and chlorine (B) quick lime and chlorine
(C) slaked lime and HCI (D) slaked lime and chlorine
79. Bleaching powder gives smell of chlorine because it
(A) is unstable
(B) gives chlorine on exposure to atmosphere
(C) is a mixture of chlorine and slaked lime
(D) contains excess of chlorine
80. Baking powder contains, baking soda and
(A) potassium hydrogen tartarate (B) calcium bicarbonate
(C) sodium carbonate (D) vinegar
81. Plaster of paris is made from
(A) lime stone (B) slaked lime (C) quicklime (D) gypsum
82. Setting of plaster of paris takes place due to -
(A) oxidation (B) reduction (C) dehydration (D) hydration.
83. Chemical formula of baking soda is
(A) MgSO4 (B) Na2CO3 (C) NaHCO3 (D) MgCO3
84. The chemical name of marble is -
(A) calcium carbonate (B) magnesium carbonate (C) calcium chloride (D) calcium sulphate
85. Washing soda has the formula -
(A) Na2CO3.7H2O (B) Na2CO3.10H2O (C) Na2CO3.H2O (D) Na2CO3
86. The raw materials required for the manufacture NaHCO3 by Solvay process are -
(A) CaCl2,(NH4)2CO3, NH3 (B) NH4Cl, NaCI, Ca(OH)2
(C) NaCI,(NH4)2CO3, NH3 (D) NaCl, NH3, CaCO3, H2O
87. Plaster of Paris hardens by
(A) giving off CO2 (B) changing into CaCO3
(C) combining with water. (D) giving out water.
88. The difference in number of water molecules in gypsum and plaster of paris is -
(A) 5/2 (B) 2 (C) 1/2 (D) 3/2
89. According to Arrhenius concept, base is a substance that
(A) gives H+ ions in solution (B) gives OH– ions in solution.
(C) accepts electrons (D) donates electrons
90. According to Bronsted - Lowry concept an acid is a substance which
(A) accepts proton (B) gives an electron pair
(C) gives proton (D) combines with H3O+ ions
91. According to Lewis concept, a base is a substance which :-
(A) donates an electron pair. (B) accepts an electron pair.
(C) produces hydronium ions. (D) combines with OH– ions.
92. The strength of the acid depends on the
(A) number of hydrogen atoms present in the molecule:'
(B) oxygen content.
(C) density.
(D) concentration of hydrogen ions furnished by ionisation.
93. Which among the following qualifies as a Lewis acid ?
(A) NaF (B) NaCl (C) BF3 (D) H3O+
94. Which of the following will qualify as a Lewis base ?
(A) BCl3 (B) CH4 (C) Cl2 (D) NH3
95. NH4+ ion in aqueous solution will behave as
(A) a base (B) an acid (C) both acid and base (D) neutral
96. Which one of the following does not act as a Bronsted acid ?
(A) NH4+ (B) HCO3– (C) HSO3– (D) CH3COO–
97. Of the given anions, the strongest Bronsted base is
(A) ClO– (B) ClO2– (C) ClO3– (D) ClO4–
98. The compound that is not a Lewis acid is -
(A) BaCl2 (B) AICl3 (C) BCl3 (D) SnCl4
99. The numerical value of negative power to which 10 must be raised in order to express hydrogen ion concentration is equal to
(A) strength of the solution (B) pH of the solution
(C) degree of hydrolysis (D) solubility product of the electrolyte
100. Which one of the following relationship is correct
(A) pH = (B) pH = log [H+] (C) log pH = [H+] (D) pH = log