1. Any substance which possesses definite mass, occupies definite volume and offers resistance to external force is called matter.
2. An elementary substance contains only one kind of atoms.
3. A chemical substance which can be split into or built up from two or more dissimilar substances is called a compound.
4. All elements and compounds are homogeneous.
5. If the components mix up thoroughly in all proportions then it is called a homogeneous mixture.
6. If the components do not mix up thoroughly and can be demarcated by boundaries, then it is called a heterogeneous mixture.
7 . Compound formation is a chemical change.
Formation of a mixture is a physical change.
8. Compounds are formed by the combination of two or more elements which combine in a fixed ratio by weight.
In the formation of a mixture, no fixed ratio is maintained.
9. The constituent elements lose their individual properties.
The components retain their individual properties.
10. All compounds are homogeneous.
Mixtures may be homogeneous or heterogeneous.
11. Compounds have definite values for density, melting point and boiling point.
Mixtures do not have definite values for density, melting point and boiling point.
12. The proportion of the constituent elements is same.
The proportion of the components can be varied.
13. Compound formation is accompanied by heat energy changes.
Heat energy changes rarely occur in the preparation of mixtures.
14. The constituents are not easily separable.
The components are easily separable.
15. Compounds have definite molecular weights.
Mixtures do not have molecular weights.
All the matter around us is not pure. The matter around us is of two types : pure substances and mixtures.
Pure Substances
A pure substance is one which is made up of only one kind of particles. All the element and compounds are pure substances because
they contain only one kind of particles like hydrogen, oxygen,
chlorine, iodine, carbon, iron, copper, silver, gold are pure substances. A pure substance cannot be separated into other kinds of matter by any physical process.
Impure Substances : Mixtures
A mixture is one which contains two or more different kinds of particles (atoms or molecules). All the mixtures are impure substances because
they contain more than one kind of particles. Examples of the mixtures are : Salt solution, sugar solution, milk, sea-water.
All the matter can be divided into three general classes : elements, compounds and mixtures.
An element is a substance which cannot be split up into two or more simpler substances by the usual chemical methods of applying heat,
light or electric energy.
An element cannot be split up into two (or more) simpler substances because it is made of only one kind of atoms.
For example, copper metal is made of only one kind of atoms called 'copper atoms'. Every substance in this world is made up of one or more of these elements.
Elements can be solids, liquids or gases. For example, sodium and carbon elements are solids, mercury and bromine elements are liquids,
whereas hydrogen and oxygen elements are gases.
Hydrogen is an element because it cannot be split up into two or more simpler substances by the usual methods of carrying out chemical reactions by
applying heat, light or electricity.
Element Symbol
Aluminium Al
Arsenic As
Barium Ba
Bromine Br
Cadmium Cd
Calcium Ca
Chlorine Cl
Chromium Cr
Cobalt Co
Fluorine F
Hydrogen H
Iodine I
Magnesium Mg
Manganese Mn
Nitrogen N
Oxygen O
Phosphorus P
Sulphur S
Uranium U
Zinc Zn
Metals Non-Metals and metalloids
All the elements can be divided into three groups :
1. Metals, 2. Non-metals, and 3. Metalloids
A metal is an element that is melleable and ductile, and conducts electricity. Examples of metals are : Iron, Copper, Aluminium, Zinc, Silver, Gold.
All the metals are solids except one metal mercury, which is a liquid.
Properties of Metals
1. Metals are Melleable. This means that metals can be beaten into thin sheets with a hammer (without breaking). For example, silver
metal can be hammered into thin silver foils because of its high malleability.
2. Metals are ductile. This means that metals can be drawn (or stretched) into thin wires. All the metals are not equally ductile. Some are
more ductile than the other. For example,
just 100 milligrams of a highly ductile metal like silver can be drawn into a thin wire about 200 metres long.
3. Metals are good conductors of heat and electricity. This means that metals allow heat and electricity to pass through them easily.
Silver metal is the best conductor of heat.
It has the highest thermal conductivity. Silver metal is the best conductor of heat. The cooking utensils and water boilers, etc., are
usually made of copper or aluminiummetals because
they are very good conductors of heat. Silver metal is the best conductor of electricity. The electric wires are made of copper and
aluminium metals because they are very good conductors of electricity.
4. Metals are Iustrous (or shiny), and can be polished. The property of a metal of having a shining surface is called metallic lustre (chamak).
The shiny appearance of metals makes them useful
in making jewellery and decoration pieces For example, gold and silver are used for making jewellery because they are bright and shiny.
The shiny surface of metals makes them good reflectors of light.
Silver metal is an excellent reflector of light.
5. Metals are generally hard Most of the metals are hard. But all the metals are not equally hard. The hardness varies from metal
to metal they can not cut with a knife.
(except sodium and potassium which are soft metals) (except sodium and potassium which are soft metals).
6. Metals are usually strong. This means that metals can hold large weights without breaking. They have high tensile strength. For example,
iron metal (in the form of steel) having a high tensile strength. Due to this iron metal is used in the construction.
7. Metals are solids at the room temperature (except mercury which is a liquid metal). All the metals like iron, copper,
aluminium, silver and gold, etc., are solids at the room temperature.
Only one metal, mercury, is in liquid state at the room temperature.
8. Metals generally have high melting points and boiling points. This means that most of the metals melt and vaporise at high
temperatures. Some exceptions, Sodium and potassium metals have low melting points.
9. Metals have high densities. This means that metals are heavy substances. For example, the density of iron metal is 7.8 g/cm3 which is
quite high. Some exceptions. Sodium and potassium metals have low densities.
10. Metals are sonorous. This means that metals make a ringing sound when we strike them. For example, Plate type musical instruments
like cymbals (manjira), and wires (or strings) for stringed musical instruments such as violin, guitar, sitar and tanpoora, etc
² NON METALS
A non-metal is an element that is neither malleable nor ductile, and does not conduct electricity. Examples of non-metals are : Carbon,
Sulphur. Oxygen, Nitrogen, Fluorine, Chlorine.
All the non-metals are solids or gases, except bromine which is a liquid non-metal at room temperature.
Properties of Non-Metals
1. Non-metals are not malleable. Non-metals are brittle. For example, sulphur and phosphorus are solid non-metals which are not malleable,
they cannot be beaten into thin sheets with a hammer.
2. Non-metals are not ductile. For example, sulphur and phosphorus are non-metals and they are not ductile.
3. Non-metals are bad conductors of heat and electricity. For example, sulphur and phosphorus are non-metals which do not conduct heat or electricity.
Some exceptions. Diamond is a non-metal which is a good conductor of heat. Graphite is a non-metal which is a good conductor of electricity.
4. Non-metals are not Iustrous. They are dull in appearance. An exception. Iodine is a non-metal having Iustrous appearance.
5. Non-metals are generally soft (except diamond which is extremely hard non-metal).
6. Non-metals are not strong. They have low tensile strength. For example graphite is a non-metal which is not strong. It has a low tensile strength.
7. Non-metals may be solid, liquid or gases at the room temperature.
8. Non-metals have comparatively low melting points and boiling points (except graphite which is a non-metal having a very high melting point 3700ºC).
9. Non-metals have low densities. For example, sulphur is a solid non-metal having a low density of 2g/cm3. One non-metal iodine has, however, high density.
10. Non-metals are not sonorous.
² METALLOIDS
The elements which show some properties of metals and some other properties of non-metals are called metalloids.
The important examples of metalloids are : Boron (B), Silicon (Si), and Germanium (Ge).
MIXTURES
A mixture is a substance which consists of two or more elements or compounds not chemically combined together. All the solutions are mixtures.
Some of the examples of mixtures are : Air, Gunpowder, Milk, Sea-water, Ink.
Types of Mixtures
1. Homogeneous mixtures, and
2. Heterogeneous mixtures
1. Homogeneous mixtures: Those mixtures in which the
substances are completely mixed together and are indistinguishable from one another,
are called homogeneous mixtures. All the homogeneous mixtures are called solutions.
Examples of homogeneous mixtures: Sugar solution, Salt solution, Sea-water, Alcohol and water mixture, Petrol and oil mixture.
2. Heterogeneous mixtures: Those mixtures in which the substances remain separate and one substance is spread throughout the other substance as small particles, droplets or bubbles,
are called heterogeneous mixtures. The suspensions of solids in liquids are also heterogeneous mixtures. A mixture containing
two (or more) immiscible liquids is also a heterogeneous mixtures. A
ll the suspensions and colloids are heterogeneous mixtures. Examples of heterogeneous mixtures are : Sugar and sand mixture, Salt and
sand mixture, Milk, Soap solution, Blood. Most of the mixtures are heterogeneous,
only solutions and alloys are homogeneous mixtures.
A compound is a substance made up of two or more elements chemically combined in a fixed proporton by mass. For example,
water (H2O) is a compound made up of two elements
, hydrogen and oxygen, chemically combined in a fixed proportion of 1:8 by mass.
Some more examples of compounds are : Ammonia (NH3), Carbon dioxide (CO2), Ice (H2O), Steam (H2O). Compounds can be
further divided into three classes : acids,
bases and salts, on the basis of their properties.
Differences between mixture and coumpounds
The 'substance which is dissolved' in a liquid to make a solution is called 'solute',
and the liquid' in which solute is dissolved is known as 'solvent'. For example, salt solution
is made by dissolving salt in water, so in salt solution, 'salt' is the 'solute' and 'water' is the 'solvent'.
A solution is a homogeneous mixture of two (or more substances). Some common examples of solutions are : Salt solution, Sugar solution, Vinegar, Metal alloys and Air.
Salt solution and sugar solution are also known as true solutions because in these solutions the particles of salt and sugar are
mixed so well with water that we cannot distinguish one from the other.
Only soluble substances form true solutions.
A sugar solution does not scatter a beam of light passing through it and render its path visible. (Because the sugar particles present in it are
so small that they cannot reflect light rays falling on them).
Properties of a Solution
1. A solution is a homogeneous mixture.
2. The size of solute particles in a solution is extremely small. It is less than 1 nm in diameter
(1 nanometre = 10–9 metre).
3. The particles of a solution cannot be seen even with a microscope.
4. The particles of a solution pass through the filter paper.
5. The solutions are very stable.
6. A true solution does not scatter light (This is because its particles are very, very small).
The various types of solutions are :
1. Solution of Solid in a Solid. Metal alloys are the solutions of solids in solids. For example, brass is a solution of zinc in copper.
2. Solution of Solid in a Liquid. Sugar solution and salt solution are the solution of solids in liquids.
3. Solution of Liquid in a Liquid. Vinegar is a solution of acetic acid (ethanoic acid) in water.
4. Solution of Gas in a Liquid. Soda-water is a solution of carbon dioxide gas in water.
5. Solution of Gas in a Gas. Air is a solution of gases like oxygen, argon, carbon dioxide and water vapour, etc.
Saturated solution :–
A solution which at a given temperature dissolves as much solute as it is capable of dissolving,is said to be a saturated solution.
Ex. At 30°C, 55 g of common salt dissolves in 100g of water. However, if more of common salt is added to the above solution, it just does not dissolve.
In such a situation, the solution of common salt containing 55 gm of salt in 100 gm of water, is a saturated solution at 30°C.
--> If a saturated solution at some particular temperature is heated, the solution becomes
unsaturated, because of the increase in solubility.
--> If a saturated solution at some higher temperature is cooled, it remains saturated. The excess solute comes out of the solution and
deposits it self in the form of crystals.
Unsaturated solution :–
When the amount of solute contained in a solution is less than the saturation level, the solution is said to be an unsaturated solution.
Ex. At 30°C, if 45 g of common salt is dissolved in 100 g of water, such solution so formed is
capable of dissolving more of the common salt, then such a solution is called unsaturated solution.
Super saturated solution :–
A solution which contains more of the solute than required to make a saturated solution, is called a super saturated solution.
Those substances which are insoluble in water form suspensions. A suspension is a heterogeneous mixture in which the small particles of a solid are spread
throughout a liquid without dissolving in it. Examples of suspensions are : Chalk-water mixture, Muddy water, Milk of magnesia, Sand particles.
To Study the Properties of a Suspension
If a beam of light is passed through a chalk and water suspension, it scatters the beam of light and renders its path visible inside it.
Properties of a Suspension
1. A suspension is a heterogeneous mixture.
2. The size of solute particles in a suspension is quite large. It is larger than 100 nm in diameter.
3. The particles of suspension can be seen easily.
4. The particles of a suspension do not pass through a filter paper.
5. The suspensions are unstable.
6. A suspension scatters a beam of light passing through it (because its particles are quite large).
A colloid is a kind of solution in which the size of solute particles is intermediate between those in true solutions and those in suspensions.
Some of the examples of colloids (or colloidal solutions) are : Soap solution, Starch solution, Milk, Ink, Blood, Jelly and Solutions of synthetic detergents.
It we shake some soap power with water in a beaker, we get a colloidal soap solution which is not perfectly transparent. If the soap
solution is kept for some time,
the soap particles do not settle down. If we filter the soap solution, the whole solution passes through the filter paper and no residue is left behind.
All these observations indicate that soap and water mixture is a true solution. The scattering of light by a soap solution and the examination
of soap solution under a high power microscope,
however, show that soap solution is not a true solution.
In a true solution (like sugar solution), the solute particles are so small that they cannot scatter (or reflect) light rays falling on them.
In a colloidal solution (or colloid), the particles are big enough to scatter light. The scattering of light by colloidal particles is known as Tyndall effect.
1. A colloid (or colloidal solution) appears to be homogeneous but actually it is heterogeneous.
2. The size of particles in a colloid is bigger than those in a true solution but smaller than those in a suspension. It is between 1 nm and 100 nm in diameter.
3. The particles of most of the colloids cannot be seen even with a microscope.
4. The particles of a colloid can pass through a filter paper. So, a colloid cannot be separated by filtration.
5. The colloids are quite stable. The particles of a colloid do not separate out on keeping.
6. A colloid scatters a beam of light passing through it colloids cannot be separated by the process of filtration, but a special technique known as centrifugation
can be used to separate the colloidal particles from a colloidal solution.
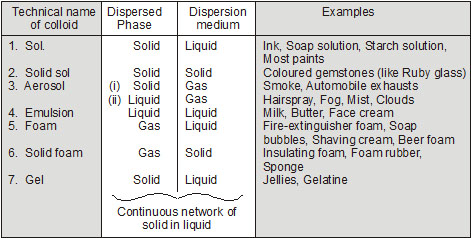
Colloids are classified according to the physical state of dispersed phase and the dispersion medium (solvent).
²
The colloidal particles are moving at random in a zigzag motion in all directions.
This type of zig-zag motion of colloidal particles is called Brownian movement. This is shown
figure. The brownian movement is caused by the collision (hitting) of the colloidal particle
with the molecules of the dispersion medium.
Tyndall effect :
The phenomenon due to which the path of light becomes visible, due to scattering of light by the colloidal particle is called Tyndall effect.
Example :
8 Tyndall effect can also be observed when a fine beam of light enters a room through a small hole. This happens due to the scattering
of light by the particles of dust and smoke in the air.
8 Tyndall effect can be observed when sunlight passes through the canopy of a dense forest. In the forest mist contains tiny droplets
of water, which act as particles of colloids dispersed in air.
The concentration of a solution is the amount of solute present in a given quantity of the solution. The most common way
of expressing the concentration of a solution is the 'percentage method'.
The concentration of a solution is defined as the mass of solute in grams present in 100 grams of the solution. The concentration
of a solution refers to the mass of solute
in 100 grams of the solution and not in 100 grams of the solvent. Concentration of solution = Mass of solute/Mass of solution *100
The Case of a Liquid Solute Dissolved in a Liquid Solvent
The concentration of a solution is defined as the volume of solute in mililitres present in 100 mililitres of the solution. The concentration
of solution refers to the volume of liquid solute in 100 mL of solution and not in 100 mL of solvent
Concentration of solution = Volume of solute/Volume of solution *100
A solution in which more quantity of solute can be dissolved without raising its temperature, is called an unstaturated solution.
A Solution in which no more solute can be dissolved at that temperature, is called a saturated solution.
If a saturated solution at a particular temperature is heated to a higher temperature, then it becomes unsaturated. Because
the solubility of solute increases on heating.
If a saturated solution available at a particular temperature is cooled to a lower temperature, then some of its dissolved solute
will separate out in the form of solid crystals.
The maximum amount of a solute which can be dissolved in 100 grams of a solvent at a specified temperature is known as the solubility
of that solute in that solvent (at the temperature).For example:
A maximum of 32 grams of potassium nitrate can be dissolved in 100 grams of water at 20ºC, therefore, the solubility of potassium
nitrate in water is 32 grams at 20ºC.
Physical Changes
Those changes in which no new substances are formed, are called physical changes. Some common examples of physical changes are :
Melting of Ice, Freezing of water,
Boiling of water, Condensation of steam, Making a solution, Glowing of an electric bulb and Breaking of a glass tumbler.
The physical changes are temporary changes which can be reversed easily to form the original substance.
Chemical Changes
Those changes in which new substances are formed, are called chemical changes. Some common examples of chemical changes
are : Burning of a magnesium wire
; Burning of paper ; Rusting of iron ; Formation of curd from milk ; and Cooking of food.
1. No new substance is formed in a physical change.
2. A physical change is a temporary change.
3. A physical change is easily reversible.
4. Very little heat (or light) energy is usually absorbed or given out in a physical change.
5. The mass of a substance does not alter in a physical change.
1. A new substance is formed in a chemical change.
2. A chemical change is a permanent change.
3. A chemical change is usually irreversible.
4. A lot of heat (or light) energy is absorbed or given out in a chemical change.
5. The mass of a substance does alter in a chemical change.
1. Separation by a Suitable Solvent
A mixture of sugar and sand can be separated by using water as solvent. The mixture of sugar and sand is taken in
a beaker and water is added to it.
The mixture is stirred to dissolve the sugar in water. The sand remains undissolved.
The sugar solution containing sand is filtered by pouring over a filter paper kept in a funnel.
A mixture of sulphur and sand can be separated by using carbon disulphide as solvent.
2. Separation by Sublimation
The changing of a solid directly into vapours on heating, and of vapours into solid on cooling is called sublimation.
To Separate a Mixture of Common Salt and Ammonium Chloride
We can separate ammonium chloride from a mixture of 'common salt and ammonium chloride' by the process of sublimation.
The mixture of common salt and ammonium chloride is taken in a china dish. The china dish is covered with an inverted glass funnel. A
loose cotton plug is put in the upper
, open end of the funnel to prevent the ammonium chloride vapours from escaping into the atmosphere. On heating the mixture, ammonium
chloride changes into white vapours. T
hese vapours rise up and get converted into solid ammonium chloride on coming in contact with the cold, inner walls of the funnel.
Pure ammonium chloride collects on
the inner sides of the funnel in the form of a sublimate and can be removed.
3. Separation by a Magnet If a mixture contains iron as one of the constituents, it can be separated by using a magnet. For example,
a mixture of iron fillings and sulphur powder can be separated by using a magnet. Separation of Scrap Iron
In factories, scrap iron is separated from the heap of waste materials by using big electromagnets fitted to a crane.
1. Separation by Filtration
Filtration is used for separating insoluble substances from a liquid. A mixture of chalk and water is separated by filtration.
When the mixture of chalk and water is poured on the filter paper fixed in a funnel, then clear water passes through the
filter paper and collects as filtrate.
The chalk particles remain behind on filter paper as residue.
2. Separation by Centrifugation
Centrifugation is a method for separating the suspended particles of a substance from a liquid in which the mixture is rotated at a
high speed in a centrifuge.
The process of centrifugation is used in dairies to separate cream from milk. The milk is put in a closed container in big centrifuge machine.
When the centrifuge machine is switched on, the milk is rotated. Due to this, the milk separates into 'cream' and 'skimmed milk'. Thus, cream is
separated from milk by centrifugation.
3. Separation by Evaporation
Evaporation is used to separate a solid substance that has dissolved in water. The use of process of evaporation for separating a mixture is based on
the fact that liquids vaporise easily whereas solids do not vaporise easily.
The common salt dissolved in water can be separated by the process of evaporation. The solution of common salt and water is taken in a china dish and heated
gently by using a burner. The water present in salt solution will form water vapours and escape into atmosphere. The process of evaporation is used
on a large scale to obtain common salt from sea-water.
4. Purification by Crystallisation
The process of cooling a hot, concentrated solution of a substance to obtain crystals is called crystallisation.
Crystallisation is a better technique than 'evaporation to dryness' because of the following reasons :
(i) Some solids (like sugar) decompose or get charred on heating to dryness during evaporation. There is no such problem in crystallisation.
(ii) The soluble impurities do not get removed in the process of evaporation. But such impurities get removed in crystallisation.
5. Separation by Chromatography
Chromatography is a technique of separating two (or more) dissolved solids which are present in a solution in very small quantities.
There are many types of chromatography but the simplest form is the paper chromatography. By using paper chromatography,
we can separate two (or more) different substances present in the same solution.
1. Take a thin and long strip of filter paper. Draw a pencil line on it. about 3 centimetres from one end.
2. Put a small drop of black ink on the filter paper strip at the centre of the pencil line.
3. When the drop of ink has dried, the filter paper strip is lowered into a tall glass jar containing some water in its lower part. The lower end of
the paper strip should dip in water but the pencil line should remain above the water level in the jar.
4. The water gradually rises up the filter paper strip by capillary action. The dye which is more soluble in water dissolves first,
rises faster and produces a coloured spot on the paper at a higher position. In this way, all the dyes present in black ink get separated
(by forming separate different coloured spots).
The paper which its separate coloured spots is called a chromatogram the important application (or uses) of chromatography :
(i) Chromatography is used to separate solutions of coloured substances.
(ii) Chromatography is used in forensic science to detect and identify trace amounts of substances in the contents of bladder or stomach.
(iii) Chromatography is used to separate small amounts of products of chemical reactions.
6. Separation by Distillation
In order to recover both, salt as well as water, from a salt-water mixture (or salt solution), the process of distillation is used.
Distillation is the process of heating a liquid to form vapour, and then cooling the vapour to get back liquid. Distillation can be represented as :
Pure water or distilled water is made from tap water by the process of distillation.
All the mixtures containing two (or more) liquids can be separated by one of the following two methods:
(i) By the process of fractional distillation.
(ii) By using a separating funnel.
Those liquids which mix together in all proportions and form a single layer (when put in a container), are called miscible liquids.
Alcohol and water are miscible liquids. A mixture of miscible liquids is separated by the process of fractional distillation.
Those liquids which do not mix with each other and form separate layers (when put in a container), are called immiscible liquids.
A mixture of immiscib le liquids is separated by using an apparatus called separating funnel.
1. Separation by Fractional Distillation
Fractional distillation is the process of separating two (or more) miscible liquids by distillation, the distillate being collected in fractions
boiling at different temperatures
. The separation of two liquids by fractional distillation depends on the difference in their boiling points. Fractional distillation is carried out by using
a fractionating column.
In most simple terms, a fractionation column can be regarded as an arrangement for providing differnet temperature zones inside it (during distillation),
the highest temperature being at the bottom of the column and the lowest temperature near its top.
1. Fractional distillation is used to separate mixtures of miscible liquids (like alcohol-water mixture and acetone-water mixture) in the laboratory.
2. Fractional distillation is used to separate crude oil 'petroleum' into useful fractions such as kerosene, petrol and diesel, etc.
3. Fractional distillation (of liquid air) is used to separate gases of the air.
The various gases of air are separated from one another by the fractional distillation of liquid air. This separation is based on the fact that
the different gases of air have different boiling points.
2. Separation by a Separating Funnel
A mixture of two immiscible luqids can be separated by using a separating funnel. The separation of two immiscible liquids by
a separating funnel depends on
the difference in their densities. A mixture of water and kerosene can be separated by using a separating funnel.
A mixture of petrol and water can also be separated by using a separating funnel. A mixture of more than two immiscible liquids can
also be separated by using a separating funnel.
The process of cooling a hot, concentrated solution of a substance to obtain crystals is called crystallisation.
Crystallisation is a process that separates a pure solid in the form of its cyrstals from a solution.
Methods :
1 We take about 10 grams of impure copper sulphate and dissolve it in minimum amount of water in a china dish to make copper sulphate solution.
2 Filter the copper sulphate solution to remove insoluble impurities.
3 Heat the copper sulphate solution gently on a water bath to evaporate water and obtain a saturated solution.
This can be tested by dipping a glass rod in hot solution from time to time. When small crystals form on the glass rod, the solution is saturated. Then stop heating.
4 Allow the hot, saturated solution of copper sulphate to cool slowly.
5 Crystals of pure copper sulphates are formed, impurities remains behind in the solution.
6 Separate the copper sulphate crystals from solution by filtration and dry.
7 Crystallisation is a better technique than evaporation to dryness because of following reasons.
(i) Some solids decompose or get charred on heating to dryness during evaporation.
(ii) The soluble impurities do not get removed in the process of evaporation. But such impurities get removed in crystallisation.
Application :
8 Purification of salt that we get from sea water
9 Separation of crystals of alum (phitkari) from impure samples.
Supply of drinking water in a city :
In cities, drinking water is supplied from water works. In water works, the methods like sedimentation, decantation, loading
, filtration and chlorination etc are used to remove undesirable materials from water. The source of water supply in a city is either a nearby river
or a lake called reservoir.
The river water and lake water usually contain suspended solid substances and some germs, so, before this water can be supplied to homes, it
must be purified to remove suspended impurities as well as germs.
The purification of river water or lake water is done in the following steps.
(1) Sedimentation : The water is allowed to stand in big tanks, where heavier suspended impurities settle down
To increase the rate of sedimentation, alum is added to it. The impurities settle at the bottom.
(2) Filtration : The semi-clear water is allowed to pass through beds of sand, charcoal and gravel to remove suspended impurities.
(3) Removal of harmful - organism or sterilisation : The harmful bacteria in filtered water can cause very serious diseases such as typhoid, cholera etc
. Thus, to the filtered water bleaching powder or chlorine gas is added. This kills the micro-organism and hence the water becomes fit for drinking.
This water is directly pupmed into overhead tanks for supply to a city.
Ex.1 Choose the elements, mixture and compounds.
(i) Brass (ii) Diamond ( iii) Sand.
Sol. (i) Brass is a mixture.
(ii) Diamond is an element (it is an allotrope of carbon which is made up of carbon atoms only.)
(iii) Sand is a compound.
E x.2 Classify each of the following as element, compound or mixture
Gold, Air, Marble, Milk, Sugar
Sol. Element ¾® Gold
Compound ¾® Marble, Sugar
Mixture ¾® Air, Milk.
Ex.3 What is a solution ?
Sol. A solution is a homogeneous mixture of two or more substances.
Ex.4 How can we obtain coloured component (dye) from Blue/Black ink?
Sol. By evaporation method, we can separate dye (solid) from liquid (solvent) and by chromatography method, we can separate components of the dye.
Ex.5 How can we separate cream from milk?
Sol. By centrifugation.
Ex.6 Define (a) solute (b) solvent.
Sol. (a) Solute : A small amount of substance which dissolves in another substance. (called solvent) is called the solute.
(b) Solvent - A larger proportion component of a solution.
Ex.7 What is a mixture ? Name two types of mixtures.
Sol. (a) Mixture is a product formed by mixing two or more substances in any proportion and constituents of a mixture do not undergo any chemical
change, but they retain their properties.
(b) Depending upon the nature of the components that form a mixture, we have different types of mixtures.
(i) Homogeneous mixture.
(ii) Heterogeneous mixture.
Ex.8 Define an alloy ? Why an alloy is considered as a mixture ?
Sol. (a) An alloy is a homogeneous mixtures of metals and cannot be separated into their components by physical methods.
(b) An alloy is considered as a mixture because it shows the properties of its constituents and can have variable composition.
Ex.9 What is a concentration of a solution ?
Sol. The concentration of a solution is the amount of solute present in a given amount (mass or volume) of solution, or the amount of solute dissolved
in a given mass or volume of a solvent.
Concentration of solution = Amount of solute / Amount of solution
or
Amount of solute / Amount of solvent.
Ex.10 A solution contains 40g of common salt in 320 g of water. Calculate the concentration in terms of mass by mass percentage of the solution.
Sol. Mass of solute (salt) = 40g,
Mass of solvent (water) = 320g
We know,
Mass of solution = Mass of solute + Mass of solvent
= 40g + 320 g = 360 g
Mass percentage of solution
=
Ex.11 Which of the following matter falls in the category of a “substance” ?
(a) Ice (b) Milk (c) Iron (d) Hydrochloric acid(HCl)
(e) Calcium oxide (CaO) (f) Mercury (g) Brick (h) Wood
Sol. A substance in science is a single pure form of matter and not a mixture of several different kinds of matter. Therefore, Ice,
Iron, Hydrochloric acid (HCl), Calcium oxide (CaO) and Mercury are categorised as substances as these cannot be separated into their
constituents by simple physical process of distillation and evaporation.
Ex.12 What is the difference between aqueous solution and non-aqueous solution ? Give one example of each.
Sol.
Ex13. Differentiate between a saturated and unsaturated solution. How will you test whether a given solution is saturated or not.
Sol.
If we add more solute to a solution and it gets dissolved then the solution is unsaturated. If it does not dissolve, then the solution is saturated.
Ex.14 Given a solution of substance A’, how will you test whether it is saturated or unsaturated with respect to ‘A, at the prevailing temperature?
What is observed when a hot saturated solution of a substance is allowed to cool ?
Sol. When no more substance ‘A’ can be dissolved in the solution at a given temperature, then the solution is saturated with respect to substance ‘A’.
When a hot saturated solution of a substance is allowed to cool, crystals of substance separates out from the solution.
Ex.15 Differentiate between true solution and colloidal solution.
Sol.
Ex.16 40 g of sugar is dissolved in 250 ml of solution. Calculate the concentration of the solution in terms of mass by volume percentage of solution.
Sol. Mass of sugar = 40 g
Vol. of solution = 250 ml
\ Mass by volume percentage of solution = 40/250 = 16%.
Ex.17 What is a suspension ?
Sol. A suspension is a heterogeneous mixture of a solid in a liquid. A suspension is formed when a liquid contains small solid particles which are not
soluble in it but are visible to naked eyes
. If such a mixture is left undisturbed, the solid particles slowly settle down to the bottom under the effect of gravity. If the particle size of
the solid is greater than 10–7 suspended in the liquid. This is called a suspension. For example, mud water, sand water, coarse lime stone particles in water.
Ex.18 What are colloidal solutions ? Give examples.
Sol. A colloidal solution is said to be intermediate state of the solution and the suspension. It is neither a true solution nor a suspension
. In colloidal solution, the particle size is between 1 nm to 100 nm. For example, a solution of soap in water, milk, blood, writing inks are some
of the colloidal solutions. T
hough these particles are 10 to 1000 times the size of a single small molecule, these are not visible to naked eyes. But these are large enough to scatter the light tha
t passes through the dispersion medium. The medium of colloidal solution is known as dispersion phase (dispersion medium) and the particles dissolved are
called dispersed phase. In milk, water is dispersion phase and fats, proteins etc. are dispersed phase. In fog, water droplets form the dispersed phase and air is dispersing medium.
Ex.19 What is Tyndall effect ? Why is Tyndall effect observed when sun light passes through dense forest.
Sol. The illumination of beam of light due to scattering on collision with certain particles is called Tyndall effect.
In dense forest, particularly in the early morning, there is fog, i.e., tiny water droplets suspended in air. These droplets are large enough to scatter beam of light
. This is why Tyndall effect can be observed when sunlight passes through dense forest.
Ex.20 What types of mixtures can be separated by simple distillation ?
Sol. Simple distillation can be used to separate the following mixtures :
(a) Two miscible liquids that boil without decomposition and have sufficient difference (> 20ºC) in their boiling points.
(b) A mixture/solution of a solid in a liquid.
Q.1 What is meant by a pure substance?
Ans. A pure substance Is one that cannot be separated into different constituents by physical or chemical process. A pure substance is one that
contains particles of only one type of a substance.
Q.2 Ust the points of differences between homogeneous and heterogeneous mixtures.
Ans. Homogeneous mixture :
(i) The composition of a homogeneous mixture is the same throughout. For example, if you make a solution of sugar in water and taste it by taking a
spoonful of solution either from the surface or from somewhere underneath the surface, it tastes equally sweet.
(ii) A homogeneous mixture has no distinct boundaries, i.e., it consists of only one phase which may be solid, liquids or gaseous.
For example, alloys such as brass (30% zinc and 70% copper) is a homogeneous mixture in the solid state. A soiution of sugar or common salt in water
is homogeneous mixture in
the liquid state. Similarly, a solution of water and alcohol is a homogeneous mixture in the liquid state. Pure air (without dust particles and suspended impurities) is a homogeneouS mixture in the gaseous state.
Heterogeneous mixture :
(i) The composition of a heterogeneous mixture is not the same throughout. For example, if we prepare a mixture of starch and sugar by thoroughly grinding it
and taste it by picking up a few particles from the various portions of the mixture, it will not have the same sweetness.
(ii) A hetetogeneous mixture has distinct boundaries d separation, i.e., itconslsts of two or more phases which can either be solids or liquids but not gaseous.
For example, iron fiilings (greyish in colour) and sulphur powder (yellow) on mixing form a heterogeneous mixture (greyish yellow). When we examine this mixture under a microscope
, we clearly see that every small portion of the mixture consists of two solid phases -one of greyish colour consisting of iron filings and the other of yellow
.colour consisting of sulphur powder. Similarly, a mixture of oil in water isf.eterogeneous mixture, consisting of two liquid phases -one of oil and the other of water.
In other words, oil in water has a distinct boundary separating oil from water.
Q.3 Differentiate between homogeneous and heterogeneous mixtures with examples.
Ans. Homogeneous mixture :
They have uniform compositions. The components of homogeneous mixtures are not physically distinct. Most solutions are homogeneous mixtures.
Salt in water, sugar in water are examples of homogeneous mixtures.
Heterogeneous mixture :
They contain physically distinct parts and have non-uniform compositions. Mixtures of sodium chloride and iron filings, salt and sulphur,
and oil and water are examples of heterogeneous mixtures. Suspensions and colloids are also heterogeneous mixtures.
Q.4 To make a saturated solution, 36 9 of sodium chloride is dissolved in 100 9 of water at 293 K. Find its concentration at this temperature.
Ans. Concentration =
Here, mass of solute = 36 g
and mass of solvent = 100g
Therefore, Mass of solution = 100 + 36 = 136 g
Thus, concentration =
Q.5 How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25°C), which are miscible with each other.
Ans. The process of distillation is used for the separation of components of a mixture containing two miscible liquids that boil without decomposition
and have sufficient difference in their boiling points (that is greater than 25°C).
Q.6 Name the technique to separate,
(i) Butter from curd, (ii)Salt from sea-water, (iii) Camphor from salt.
Ans. (i) Centrifugation (ii) Evaporation (iii) Sublimation
Q.7 What type of mixtures qre separated by the technique of crystallisation?
Ans. The crystallisation method is used to purify solid § mixtures.
Q.8 Classify the following as chemical or physical changes :
• Cutting of trees
• Melting of butter in a pan
• Rusting of almirah
• Boiling of welter to form steam
• Passing of electric current through water and the water breaking down Into hydrogen and oxygen gases
• Dissolving common salt in water
• Making a fruit salad with raw fruits, and
• Burning of paper and wood.
Ans. Physical changes: Cutting of trees, melting of butter in a pan, boiling water to form steam, making a fruit salad with row fruits, dissolving common salt in water.
Chemical changes: Rusting of almirah, passing of electric current through water and the water breaking down into hydrogen and oxygen gases,
burning of paper and wood.
Q.9 Try segregating the things around you as pure substances or mixtures: (a) distilled water (b) curd
(c) diamond (d) ice-cream (e) kerosene oil (f) cooking oil (g) stee l (h) graphite (i) raw rubber
(j) wlcanized rubbe (k) solder wire (l) glass (m) iron nail.
Ans. (i) Pure substance: Water, glass, iron nail, graphite, diamond, raw rubber.
(ii) Mixture: curd, ice cream, kerosene oil, cooking oil, steel, vulcanized rubber, solder wire.
Q.10 Which separation techniques win you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride a'nd ammonium chloride.
(c) Small piece of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f) Oil from curd.
(g) Tea leaves from tea
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Ans. (a) Evaporation (b) Sublimation (c) Filtration (d) Chromatography
(e) Centrifugation (f) Distillation (g) Sieving (h) Magnetic separation
(i) Sieving and winnowing (j) Sedimentation, decantation and filtration.
Q.11 Write the. steps you would use for making tea. Use the words solUtion, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Ans. Take some water in pan. Keep the pan over flame. Add sugar and tea leaves, which are solute in the water in the pan, which is the solvent
Heat the water over the pan till the sugar, which is soluble in water dissolves in it. Tea leaves are insoluble in water and wiU remain suspended.
Now add water to the sugar and tea leaves solution and bring the mixture to a boil. Filter the prepared tea through a sieve. Filtrate should be
poured in a cup, while the residue can be thrown away.
Q.12 Pragya tested the solubility of three different substances at different temperatures and coUected the data as given below.
Result are given in the following table, as grams of.substance dissolved in 100 grams of water to form a saturated solution.
(a) Potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K ?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What
would she observe as the solution cools?
(c) Explain. Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature ?
(d) What is the effect of change of temperature on the solubility of a salt ?
Ans. (a) Since 62 9 of potassium nitrate is dissolved in 100 9 of water to prepre a saturaterd solutior at 313 K, 31 g of potassium nitrate should b
e dissolved in 50 g of water to prepare a saturated solution at 313 K.
(b) The amount of potassium chloride that should be dissolved in water to make a saturated solution increases with temeprature.
Thus, as the solution cools some of the potassium chloride will precipitate out of the solution.
(c) The solubility of the salt at 293 K are
Potassium nitrate - 32 g
Sodium chloride - 36 g
Potassium chloride - 35 g
Ammonium chloride - 37 g
Ammonium chloride has the highest solubility at 293 K.
(d) The solubility of a salt increases with temperature.
Q.13 Explain the following by giving examples.
(a) Saturated solution (b) Pure substance (c) Colloid (d) Suspension
Ans. (a) At any particular temerature, a solution that has dissolved as much solute as it is capable of dissolving is said to be saturated solution
. Few examples of saturated solutions are soft drinks and nitrogen in Earth’s soil.
(b) A pure substance is one that cannot be separated into different constituents by physical or chemical processes.
A pure substance is one that contains particles of only one type of a substance. Pure substance can be elements or compounds.
Some examples of pure substances are iron, water, oxygen, etc.
(c) Colloids are heterogeneous mixtures in whiCh the particle size is too small to be seen with the naked eye, but is big enough to scatter light.
Smoke, paint, butter are few examples of colloids.
(d) Materials that are insoluble in a solvent and have particles that are visible to naked eyes form a suspension. A suspension is a heterogeneous mixture
. Some examples of suspension are water with chalk particles, sandy water and water with stones.
Q.14 Classify each of the following as a homogeneous or heterogeneous mixture.
Soda, water, wood, air, soil, vinegar, filtered tea.
Ans. Homogeneous - soda, water, air, vinegar, filtered tea.
Heterogeneous - wood, soil.
Q.15 How would you confirm that a colourless liquid given to you is pure water?
Ans. If the boiling and freezing points of the given liquid comes out to be 100°C and 0°C respectively under one atmosphere pressure, it confirms
that the given liquid is pure water.
Q.16 Identify the solution among the following mixtures.
(a) Soil (b) Sea water (c) Air (d) Coal (e) Soda water.
Ans. Sea water, air, and soda water are solutions.
Q.17 Which of the following will show “Tyndall effect”?
(a) Salt solution (b) Milk (c) Copper sulphate solution (d) Starch solution
Ans. Colloids show Tyndall effect, Milk is a colloid. Thus, it will show Tyndall effect. Therefore the correct answer is (b).
Q.18 Classify the following into elements, compounds and mixtures.
(a) Sodium (b) Soil (c) Sugar solution (d) Silver (e) Calcium carbonate
(f) Tin (g) Silicon (h) Coal (i) Air (j) Soap (k) Methane (l) Carbon dioxide (m) Blood
Ans. Elements: sodium, silver, tin and silicon
Compounds: calcium carbonate, soap, methane and carbon dioxide.
Mixtures: soil, sugar solution, coal, air and blood.
Q.19 Which of the following are chemical changes?
(a) Growth of a plant (b) Rusting of iron (c) Mixxing of iron filings and sand (d) Cooking of food
(e) Digestion of food (f) Freezing of water (g) Burning of a candle
Ans. Rusting of iron, cooking of food, digestion of food, and burning of candle are chemical changes.
Q. 1 What would you observe when
(a) a saturated solution of potassium chloride prepared at 60ºC is allowed to cool to room Temperature.
(b) an aqueous sugar solution is heated to dryness
(c) a mixture of iron filings and sulphur powder is heated strongly.
Q. 2 A child wanted to separate the mixture of dyes constituting a sample of link. He marked a line with the link on a filter paper and placed
the filter paper in a glass containing water as shown In the figure. The filter paper was removed when the water moved near the top of the filter paper.
(a) What would you expect to see, if the ink contains three different coloured components?
(b) Name the technique used by the child.
(c) Suggest one more application of this technique.
Q.3 (a Under which category of mixtures will you classify alloys and why ?
(b) A solution is always a liquid. Comment.
(c) Can a solution be heterogeneous ?
Q.4 On heating calcium carbonate gets converted to calcium oxide and carbon dioxide.
(a) Is this a physical or a chemical change
(b) Can you prepare one acidic and one basicsolution by using the products formed in the above process ? If so, write the chemical equation involved.
Q.5 Give three differences between a colloidal solution and a true solution.
Q.6 A group of student took an old shoe box and covered it with black paper from all side.
They fixed a source of light ( a torch) at one end of the box by making a hole in it and made another hole on the other side to view the light.
They placed a milk sample contained in a beaker/tumbler in the box milk taken in the tumbler was illuminated. They tried the same activity by
taking a salt solution but found that light simply passed through it ?
(a) Explain why the milk sample was illuminated. Name the phenomenon involved.
(b) Same results were not observed with a salt solution. Explain.
(c) Can you suggest two more solution which would show the same effect as shown by the milk solution ?
Q.7 You are provided with a mixture containing sand, iron fillings ammonium chloride and sodium Chloride
. Describe the procedures you would use to separate these constituents from the Mixture?
Q.8 What is meant by fractional distillation. How is it different from simple distillation. Give one Example of fractional distillation in industry.
Q.9 What is a colloidal solution ? Haw is it different from a true solution. Identify the colloidal solutions from the following :
Copper sulphate solution, vinegar, blue ink, milk of magnesia.
Q 10 A solution contains 110 g of sugar in 500 g of water. Calculate the concentration in terms of mass by mass percentage of the solution.
Q.11 Identify physical and chemical changes from the following :
Rusting of iron, cooking of food, freezing of water, burning of candle, melting of wax, glowing of a bulb.
Q.12 Distinguish between a mixture and a compound.
Q.13 (a) 4 g of solute dissolved in 36 g of water. What is the mass percentage of the solution ?
(b) How can we make a saturated solution, unsaturated ?
Q.14 Name the principle used to separate kerosene and water. Draw a neat and labelled diagram of the apparatus used in this separation.
Q. 15 (a) Name the technique that you will apply for the separation of the following
(i) Pigments from an extract of flower petals.
(ii) Cream from milk
(b) Mention any two differences between metals and non - metals
Q.16 Write two differences between colloids and the suspension on the basis of their characteristics. Identify the colloids from the following :
Copper sulphate solution, milk, soluton of sugar and smoke
Q. 17 Give the difference between :
(a) a physical and a chemical change
(b) a homogeneous and a heterogeneous mixture
(c) a colloid and a true solution
Q.18 Which separation techniques will you apply for the separation of the following ?
(a) Mixture of iron and sulphur
(b) Camphor from the mixture of camphor and sodium chloride
(c) Different components of ink
Q.19 Mention the difference between true solution suspension and colloidal solution on the basis of filteration and stability.
Q.20 Define the terms solute, solvent and solution. When is a solution said to be saturated?
State two ways by which a saturated sugar solution can be made unsaturated.
Q.1 Milk is a _______ solution while vinegar is a ______ solution.
(A) suspension, colloidal
(B) colloidal, suspension
(C) true, colloidal
(D) colloidal, true
Q.2 A liquid and a solid together consisting a single phase is known as:
(A) solution (B) solute (C) solvent (D ) emulsion
Q.3 Which of the following is a homogeneous system?
(A) Muddy water
(B) Bread
(C) Concrete
(D) A solution of sugar in water
Q.4 The zig-zag movement of dispersed phase article in a colloidal system is known as:
(A) transitional motion
(B) circular motion
(C) linear motion
(D) brownian motion
Q.5 An emulsion is a colloidal system of:
(A) solid dispersed in solid (B) liquid dispersed in liquid
(C) gas dispersed in liquid (D) liquid dispersed in solid
Q.6 Milk is :
(A) fat dispersed in water (B) fat dispersed in milk
(C) fat dispersed in fat (D) water dispersed in milk
Q.7 Scattering of light takes place in :
(A) electrolytic solutions (B) colloidal solutions
(C) electrodialysis (D) electroplating
Q.8 Foam is a colloidal solution of :
(A) gaseous particles dispersed in gas (B) gaseous particles dispersed in liquid
(C) solid particles dispersed in liquid (D) solid particles dispersed in gas
Q.9 Which of the following forms a colloidal solution in water?
(A) Salt (B) Glucose (C) Starch (D) Barium nitrate
Q.10 Movement of colloidal particles under the influence of electrical field is called:
(A) electrophoresis (B) dialysis (C) ionisation (D) electrodialysis
Q.11 Gelatin is also called as:
(A) protective colloid
(B) hydrophilic colloid
(C) emulsion
(D) none of these
Q.12 The sky looks blue due to :
(A) dispersion effect (B) reflection (C) scattering (D) transmission
Q.13 In colloidal state, particle size ranges from:
(A) 1 to 10 A0 (B) 20 to 50 A0 (C) 10 to 100 A0 (D) 1 to 280 A0
Q.14 Tyndall effect is observed in:
(A) solution (B) precipitate (C) sol ( D) vapour
Q.15 Brownian movement is due to:
(A) temperature fluctuations within the liquid phase
(B) attraction and repulsion between the charges on the colloidal particles
(C) impact of molecules of the dispersion medium on the colloidal particles
(D) convention currents
Q.16 Difference between a crystalloid and a colloid is in :
(A) particle size (B) the nature of solute
(C) diffusion through a membrane (D) all of the above
Q.17 Blood is _____ charged sol.
(A) negatively (B) positively (C) neutral (D) none of these
Q.18 Ice cream is an example of:
(A) true solution (B) emulsion (C) colloid (D) suspension
Q.19 Water loving colloids are called:
(A) hydrophobic colloids (B) reversible colloids
(C) irreversible colloids (D) hydrophilic colloids
Q.20 The technique used in ultra microscope is:
(A) adsorption (B) coagulation (C) Tyndall effect (D) electrophoresis
Q.21 Coagulation occurs due to:
(A) the scattering of light (B) the presence of charges
(C) the neutralization of charges (D) unequal bombardment by solvent molecules
Q.22 Sol is:
(A) solid dispersed in liquid (B) liquid dispersed in gas
(C) gas dispersed in liquid (D) gas dispersed in solid
Q.23 In both dialysis and osmosis which particles do not pass through the semi-permeable membrane?
(A) Water (B) Small molecules (C) Colloids (D) All of these
Q.24 The separation of colloidal particles from those of molecular dimensions is called:
(A) dialysis (B) pyrolysis (C ) peptization (D) photolysis
Q.25 Electrophoresis is due to :
(A) the neutralization of charge (B) the presence of charge
(C) the scattering light (D) all of the above
Q.26 Adsorption property is applied in ;
(A) sewage disposal (B) ultramicroscope
(C) smoke precipitator (D) medicine
Q.27 Liquid dispersed in gas is called:
(A) aerosol ( B) solid sol (C) sol (D) solid foam
Q.28 Drinking soda is an example of ‘a solution of:
(A) gas in liquid (B) liquid in gas (C) gas in gas (D) soild in liquid
Q.29 Amalgam is a solution of:
(A) solid in solid (B) solid in liquid (C) liquid in solid (D) liquid in liquid
Q.30 Which of the following is a true solution?
(A) NaCl in sulphur dioxide (B) Copper in silver
(C) Salt in petrol (D) Mud in water
Q.31 Which of the following statements is correct?
(A) Compounds can be separated into constitu ents by physical processes
(B) The boiling points and melting points of compounds are not fixed
(C) The composition of compounds are not fixed
(D) The properties of compounds are entirely different from those of its constituents
Q.32 Water is:
(A) a compound (B) a mixture (C) true solution (D) all of these
Q.33 he material which contains at least two pure substances and shows the properties of their constituents is called:
(A) a compound (B) an element (C) a mixture (D) a solution
Q.34 Select the odd one.
(A) Hydrogen (B) Oxygen (C) Steam (D) Chlorine
Q.35 Milk of Magnesia is an example of:
(A) emulsion (B) true solution (C) colloid (D) suspension
Q.36 A solution of iodine in carbon tetra - chloride is known as:
(A) aqueous solution (B) alcoholic solution
(C) nonaqueous solution (D) tincture of iodine
Q.37 Solid foam is:
(A) solid dispersed in solid (B) liquid dispersed in solid
(C) gas dispersed in solid (D) solid dispersed in liquid
Q.38 What is the property used in sewage disposal?
(A) Coagulation (B) Adsorption (C) Electrophoresis (D) Tyndall effect
Q.39 Which of the following is a characteristic of both mixtures and compounds?
(A) They contain components in fixed proportions
(B) Their properties are the same as those of their components
(C) Their weight equals the sum of the weights of their components
(D) Energy is given out when they are being prepared
Q.40 When salt is dissolved in water, there is:
(A) an increase of boiling point
(B) no change in boiling point
(C) a decrease of boiling point
(D) none of the above
Q.41 How many grams of hydrochloric acid are formed when 2 grams of hydrogen combine
with excess of chlorine?
(A) 35.5 gm (B) 36.5 gm (C) 73 gm (D) 37.7 gm
Q.42 The size of a colloidal particle is :
(A) 10-1 to 10-3 cm (B) 10-5 to 10-7 cm
(C) 10-8 to 10-5 cm (D) 10-6 to 10-8 cm
Q.43 Which of the following is not a compound?
(A) Sugar (B) Common salt (C) Diamond (D) Plaster of Paris
Q.44 Which of the following is an example of a mixture?
(A) Sugar (B) Brass (C) CO2 (D) N02
Q.45 Which of the following properties is different for solids, liquids and gases?
(A) Movement of molecules (B) Particle size of the substance
(C) Mass of the substance (D) Energy exchanges
Q.46 Sublimation of iodine, camphor and naphthalene is an example of:
(A) chemical change
(B) energy change
(C) physical change
(D) irreversible change
Q.4 Which of the following obey the law of constant proportions in their formation?
(A) Mixtures (B) Compounds. (C) Elements (D) Colloids
Q.48 Which of the following is not a chemical change?
(A) Rusting of iron (B) Converting water into steam
(C) Preparing curd from milk (D) Burning of coal
Q.4 Burning of a candle is a/an :
(A) chemical change
(B) physical change
(C) energy change
(D) reversible change
Q.50 A chemical compound always consists of the same elements which combine in the same fixed ratio by weight’, is the statement of :
(A) Law of definite proportions (B) Gay-Lussac’s law
(C) Law of multiple proportions (D) None of these
Q.51 The most abundant metal in the earth’s crust is:
(A) Fe (B) Cu (C) Al (D) Au
Q.52 The number of nonmetallic elements which are liquids at room temperature is :
(A) 2 (B) l (C) 4 (D) 3
Q.53 The most abundant element in the earth’s crust is
(A) Si (B) C (C) O (D) Ca
Q.54 Which of the following is expected to conduct electricity?
(A) Diamond (B) Molten sulphur (C) Molten KCl (D) Crystalline NaCl
Q.55 Diamond is the strongest naturally occurring substance. Its hardness is due to:
(A) high solubility in water (B) covalent bonds
(C) high electrical conductance (D) high boiling point
Q.56 Which of the following is an insulator?
(A) Aluminium (B) Diamond (C) Graphite ( D) Silicon
Q.57 Paramagnetic substances are:
(A) strongly magnetic
(B) weakly magnetic
(C) nonmagnetic
(D) none of these
Q.5 Which of the following is not a compound-
(A) Common salt
(B) Water
(C) Iron fillings
(D) Copper sulphate
Q.59 Which of the following is not a mixture-
(A) Soil (B) Air (C) Steam (D) Milk
Q.60 Brass contains -
(A) Gold and copper
(B) Copper and zinc
(C) Zinc and silver
(D) Copper and silver
Q.61 Which of the following is not a chemical change-
(A) Electrolysis of water
(B) Boiling of water
(C) Digestion of food
(D) Burning of magnesium ribbon in oxygen to form magnesium oxide.
Q.62 Which o f the following is a liquid metal-
(A) Copper (B) Mercury (C) Bromine (D) Silver
Q.63 Which of the following is not a pure substance-
(A) Mercury (B) Sugar (C) Blood (D) Salt
Q.64 Which of the following can be classified as a 'Substance' -
(A) Milk (B) Sea-water (C) Ice (D) Cast iron
Q.65 Which of the following gives a true solution in water-
(A) Starch (B) Sugar (C) Chalk powder (D) Egg albumin
Q.66 Which of the following statement s is not correct-
(A) A compound is a pure substance (B) Compound is homogeneous in nature (C) Compound always contains two or more elements (D) Compound can be separated into constituent elements by some physical process.
Q.67 Which of the following statements is not true-
(A) True solutions are homogeneous in nature (B) Suspensions are heterogeneous in nature (C) Solute particles in a colloidal solution can be separated by filtration
(D) True solutions are transparent to light
Q.68 Which of the following is the second most abundant metal in the earth's crust ?
(A) Copper (B) Aluminium (C) Iron (D) Zinc
Q.69 Which of the following will show Tyndall effect-
(A) Starch solution
(B) Sodium chloride solution
(C) Copper sulphate solution
(D) Sugar solution
Q.70 When a beam of light is passed through a true solution, it gets-
(A) Reflected
(B) Absorbed
(C) Scattered
(D) Path of light does not visible
Q.71 Camphor can be purified by-
(A) Distillation
(B) Filtration
(C) Sedimentation
(D) Sublimation
Q.72 Carbon burns in oxygen to form carbon dioxide. The properties of carbon dioxide are-
(A) Similar to oxygen
(B) Similar to carbon
(C) Totally different from both carbon and oxygen
(D) Much similar to both carbon and oxygen
Q.73 A mixture of common salt and water can be separated by-
(A) Sublimation
(B) Evaporation
(C) Separating funnel
(D) Filtration
Q.74 The process of cooling a hot, concentrated solution of a substance to obtain crystal is called -
(A) Fractional distillation
(B) Distillation
(C) Crystallisation
(D) Chromatography
Q.75 A solution in which more quantity of solute can be dissolved without raising its temperature is called -
(A) Unsaturated solution
(B) Saturated solution
(C) Super saturated solution
(D) Concentrate solution
Q.76 Colloidal particles can be normally seen by-
(A) Ordinary microscope
(B) Naked eye
(C) Electron microscope
(D) Telescope
Q.77 A mixture of alcohol and water can be separated by -
(A) Separating funnel
(B) Fractional distillation
(C) Distillation
(D) Crystallisation
Q.78 Which of the following is not a compound -
(A) Common salt (B) Water (C) Iron fillings (D) Copper sulphate
Q.79 Which of the following is not a mixture -
(A) Soil (B) Air (C) Steam (D) Milk
Q.80 Brass contains -
(A) Gold and copper (B) Copper and zinc
(C) Zinc and silver (D) Copper and silver
Q.81 Which of the following is not a chemical change-
(A) Electrolysis of water
(B) Boiling of water
(C) Digestion of food
(D) Burning of magnesium ribbon in oxygen to
form magnesium oxide.
Q.82 Which of the following is a liquid metal-
(A) Copper (B) Mercury (C) Bromine (D) Silver
Q.83 Which of the following is not a pure substance
(A) Mercury (B) Sugar (C) Blood (D) Salt
Q.84 Which of the following can be classified as a 'Substance' -
(A) Milk (B) Sea-water (C) Ice (D) Cast iron
Q.85 Which of the following gives a true solution in water-
(A) Starch (B) Sugar
(C) Chalk powder (D) Egg albumin
Q.86 Which of the following statements is not correct-
(A) A compound is a pure substance
(B) Compound is homogeneous in nature
(C) Compound always contains two or more
elements
(D) Compound can be separated into constituent
elements by some physical process.
Q.87 Which of the following statements is not true-
(A) True solutions are homogeneous in nature
(B) Suspensions are heterogeneous in nature
(C) Solute particles in a colloidal solution can
be separated by filtration
(D) True solutions are transparent to light
Q.88 Which of the following is the second most abundant metal in the earth's crust ?
(A) Copper (B) Aluminium (C) Iron (D) Zinc
Q.89 Which of the following will show Tyndall effect-
(A) Starch solution (B) Sodium chloride solution
(C) Copper sulphate solution (D) Sugar solution
Q.90 When a beam of light is passed through a true solution, it gets-
(A) Reflected (B) Absorbed
(C) Scattered (D) Path of light does not visible
Q.91 Camphor can be purified by-
(A) Distillation (B) Filtration
(C) Sedimentation (D) Sublimation
Q.92 Carbon burns in oxygen to form carbon dioxide. The properties of carbon dioxide are-
(A) Similar to oxygen
(B) Similar to carbon
(C) Totally different from both carbon and oxygen
(D) Much similar to both carbon and oxygen
Q.93 A mixture of common salt and water can be separated by-
(A) Sublimation (B) Evaporation
(C) Separating funnel (D) Filtration
Q.94 The process of cooling a hot, concentrated solution of a substance to obtain crystal is called
(A) Fractional distillation
(B) Distillation
(C) Crystallisation
(D) Chromatography
Q.95 A solution in which more quantity of solute can be dissolved without raising its temperature is called -
(A) Unsaturated solution
(B) Saturated solution
(C) Super saturated solution
(D) Concentrate solution
Q.96 Colloidal particles can be normally seen by-
(A) Ordinary microscope
(B) Naked eye
(C) Electron microscope
(D) Telescope
Q.97 A mixture of alcohol and water can be separated
by -
(A) Separating funnel
(B) Fractional distillation
(C) Distillation (D) Crystallisation
1. D 2. A 3. D 4. D 5. B 6. A 7. B 8. B 9. C 10. A 11. A 12. C
13. C 14. C 15. C 16. D 17. A 18. B 19. D 20. C 21. C 22. A 23. C
24. A 25. B 26. D 27. A 28. A 29. C 30. B 31. D 32. A 33. C 34. C
35. D 36. C 37. C 38. A 39. C 40. A 41. C 42. B 43. C 44. B 45. A
46. C 47. B 48. B 49. A 50. A 51. C 52. A 53. C 54. C 55. B 56. B 57.
B 58. C 59. C 60. B 61. B 62. B 63. C 64. C 65. B 66. D 67. C 68. C
69. A 70. D 71. D 72. C 73. B 74. C 75. A 76. C 77. B 78. C 79. C 80.
B 81. B 82. B 83. C 84. C 85. B 86. D 87. C 88. C
89. A 90. D 91. D 92. C 93. B 94. C 95. A 96. C 97. B