A wide variety of materials consists essentially of elements and compounds having different characteristics
exist around us. Some of them are sour, some are bitter, while some are salty in taste.
For Example – Sour and bitter tastes of food are due to acids and bases, respectively, present in them.
Acids react with bases to produce salt whose properties are different from acid and base.
The term "acid" is derived from the latin word "acidus" meaning sour to taste.
Example – Sour taste of lemon, unripened grapes, Vinegar, tomatoes etc.
* According to Arrhenius theory :
"An acid is a substance which dissolved in water, it ionizes and releases hydrogen ions [H+(aq.)] in solution".
![]()
Note :- Hydrogen ion do not exist as H+ ions in solution, they attach themselves to the polar
water molecules to form hydronium ions or hydroxonium ions, (H3OÅ or H+(aq.))
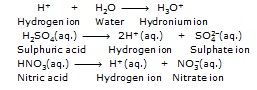
(I) On the basis of their source acids are of two type –
(i) Mineral acids (ii) Organic acids
(i) Mineral Acids (Inorganic acids) :-
The acids which are usually obtained from minerals are known as inorganic acids.
(ii) Organic Acids :-
The acids which are usually obtained from plants and animals are known as organic acids.

(II) On the Basis of their Basicity :-
"The basicity of an acid is the number of replaceable hydrogen atoms present in a molecule that can
be produced by the complete ionisation of one molecule of that acid in aqueous solution."
or
"Basicity of an acid is determined by number of hydronium ions (H3O+/H+(aq)) produced per molecule of an acid on ionisation."
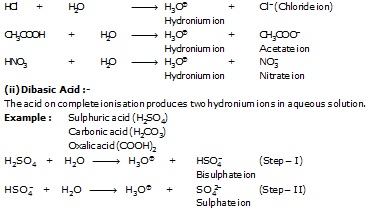
(i) Monobasic Acids :-
The acid on complete ionisation produce one hydronium ion in aqueous solution.
Example : Hydrochloric acid (HCl)
Hydrobromic acid (HBr)
Hydrofluoric acid (HF)
Hydroiodic acid (HI)
Nitric acid (HNO3)
Acetic acid (CH3COOH)
Formic acid (HCOOH)
(iii) Tribasic Acid :-
The acid on complete ionisation produces three hydronium ions in aqueous solution.
Example :
![]()
(III) Classification on the basis of their strength :-
(i) Strong Acid :-
The acid which undergoes complete ionisation in aqueous solution are known as strong acids.
Example : 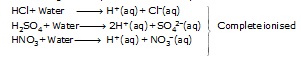
(ii) Weak Acid :-
The acid which undergoes partial or incomplete ionisation in aqueous solution are known as weak acids.
![]()
Example : Formic acid (HCOOH), Oxalic acid (COOH)2
Carbonic acid (H2CO3), phosphoric acid (H3PO4)
(IV) Classification on the basis of concentration of the Acid :-
(i) Concentrated Acid :-
The acids which contains very small amount of water is called a concentrated acid.
(ii) Dilute Acid :-
The acids which contains more amount of water is called a dilute acid.
"Strength of an acid is not depend upon the concentration of an acid"
Strength of an Acid µ Concentration of hydronium ion.

Substances with bitter taste and give a soapy touch are known as bases but many bases have corrosive nature. So bases are defined as "
* According to Arrhenius :
those substances which give hydroxide or hydroxyl ion (OH–) in their aqueous solution" are called bases.
NaOH(aq.) ® Na+(aq) + OH–(aq)
KOH(aq.) ® K+(aq) + OH–(aq)
Example – Sodium hydroxide (NaOH), Zinc oxide (ZnO), Copper oxide (CuO), Calcium hydroxide [Ca(OH)2], Aluminium hydroxide [Al(OH)3].
* The compounds which are either metallic oxides or metallic hydroxides. Which combines with acids to form salts and water only.
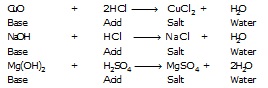
* Alkalis
Bases which completely dissolves in water are called alkalis.
Examples – KOH, NaOH, Ca(OH)2
![]()
(i) Strong alkalis or bases :-
The alkalis or bases which undergo almost complete ionisation in aqueous solution are known as strong alkalis or bases.
Examples –
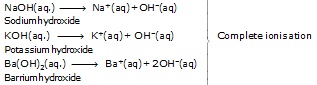
(ii) Weak alkalis or bases :-
The alkalis or bases which undergo only partial ionisation in aqueous solution are known as weak alkalis or Bases.
Example –
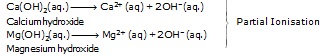
(i) Concentrated Base or Alkali –
The bases or alkalis which contain very small amount of water is called a concentrated bases or alkalis.
(ii) Dilute Base –
The bases or alkali which contain more amount of water is called a dilute bases or alkalis.
Acidity of a base is determined by the number of hydroxyl (OH–) ions produced by per molecule of a Base or Alkali on complete dissociation in water "or"
The "number of hydrogen ions of an acid with which a molecule of that alkali or base react to produce salt and water is known as acidity of an alkali or Base".
(i) Mono acidic Bases or Alkali –
The base or alkali on complete ionisation produce one hydroxyl (OH–) ion in aqueous solution.
Example – 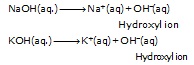
(ii) Diacidic Bases (or alkalis) –
The base or alkali on complete ionisation produce two hydroxyl ion (OH–) in aqueous solution
Example –
(A) Diacidic Bases of –
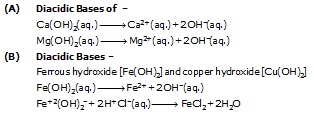
(iii) Tri Acidic Bases –
The base or alkali on complete ionisation produce three hydroxyl ion (OH)– in aqueous solution.
Example – ![]()
Q. Identify strong and weak acids as well as strong and weak bases from the following. H2CO3. CH3COOH, NaOH, NH4OH, KOH, Ca(OH)2, HCl.
(I) Physical properties of Acid –
The important properties of acids are given below
(i) Acids have a sour taste.
(ii) Acids turn blue litmus to red.
(iii) Acid solution conduct electricity (They are electrolytes).
(iv) Effect on Skin – All strong mineral acids have a corrosive action on skin and cause painful burns.
Example – Concentrated sulphuric acid stains the skin black.
Concentrated nitric acid & hydrochloric acid stains the skin yellow.
(v) Electrical Conductivity – All mineral acids are good conductors of electricity and conduct electricity in their aqueous solution. On electrolysis, they decompose liberating hydrogen at cathode.
(II) Chemical Properties of Acids
(i) Acids react with metal to form hydrogen gas
Most of the acids react with mtals to form salts and evolve hydrogen gas.
![]()
Most of the acid react with metal to form solid and evolve hydrogen gas.
Object: To show the reaction of acids on metals.
Take a test-tube. Add to it about 5 mL of dilute H2SO4 and a few pieces of zinc granules. Set up the apparatus as shown in figure.
(ii) Acids react with metal carbonates (and metal hydrogen carbonates) to form carbon dioxide gas.
(a) When dilute hydrochloric acid react with soldium carbonate then sodium chloride, carbondioxide and water is formed.
![]()
Sodium Hydrochloric sodium carbon water
carbonate acid chloride dioxide
(b) When dilute hydrochloric acid react with sodium hydrogencarbonate, then sodium chloride, carbon dioxide and water are formed.
NaHCO3(s) + HCl(aq) ¾® NaCl(aq) + CO2(g)+H2O(l)
Sodium Hydrochloric sodium carbon water
hydrogen acid chloride dioxide
carbonate
Object: To show the reaction of acids with metal carbonates and metal hydrogen carbonates.
Method:
(i) Take two test tubes and mark them 1 and 2.
(ii) Take about 0.5g of sodium carbonate (Na2CO3) in test tube 1 and 0.5g of sodium hydrogen carbonate in test tube 2.
(iii) Add about 2 mL of dilute hydrochloric acid to test tube 1 and pass the evolved gas into lime water.
(iv) Now, add about 2 mL of dilute hydrochloric acid to test tube 2 and pass the evolved gas into lime water.
Observation: When dilute hydrochloric acid is added to either sodium carbonate or sodium hydrogen carbonate, a gas is evolved which turns lime water milky, Hence the gas produced is carbon dioxide. So the chemical equations for the reaction taking place, may be written as follows:
![]()
(III) Acids react with bases (or alkalis) to form salt and water :
![]()
The reaction between an acid and a base to form salt and water is called a neutralisation reaction.
![]()
Sodium Hydrochloric Sodium Water
hydroxide acid chloride
Object: To show reaction of acids with bases.
Method:
(i) Take about 2-3 mL of dilute NaOH solution in a test-tube and add 2 drops of phenolphthalein solution (indicator) to it. the colour of solution will change to pink.
(ii) Now add dilute hydrochloric acid to it drop by drop till the pink colour of above solution disappears.
Observation: In the above activity, pink colour of solution disappears because the effect of base is nullified by the acid and vice-versa. The chemical equation for the reaction we can write as given below:
![]()
Base Acid Salt Water
Result: The above mentioned activity shows that an acid neutralizes a base in aqueous solutio. It is termed as neutrilisation reaction. We can generalise the above reactions:
(IV) Acids react with metal oxides to form salt and
water :
Acids react with metal oxides to form salt and water :
![]()
Copper(II) oxide is a metal oxide. Dilute hydrochloric acid reacts with copper(II) oxide to form copper(II) chloride and water :
CuO(s) + 2HCl(aq) ¾® CuCl2(aq) + H2O(l)
Copper Hydrochloric Copper Water
(II) oxide acid (II) chloride
(Blue Green)
Object: To show the reaction of acids on metal oxides.
Method:
(i) Take about 2g black copper (II) oxide in a beaker.
(ii) Add dilute hydrochloric acid to it slowly with stirring.
Observation: Slowly copper (II) oxide dissolves and solution of blue-green colour is formed which is copper (II) chloride. The chemical equation for the reaction is as follows:
![]()
Black Blue-Green
Result: The abouve mentioned activity shows that generally metal oxides react with acids to form salt and water.
This mineral acids causes severs burns on the skins and attack and eat up materials like clothes,wood,metal structures and stonework, so they are said to be corrosive. Acids are never stored in metal containers because they gradually corrode and eat up the metal container.
The strong bases (or alkalies) such as sodium hydroxide are also very corrosive, and attack and destroy our skin.
² What is common in all acids?
An acid is a substance which dissociates (on ionises) on dissolving in water to produce hydrogen ion
[H+ (aq) ions].
![]()
Hydrochloric acid Hydrogen ions Chloride ions
A common thing in all the acids is that they produce hydrogen ions [H+ (aq) ions] when dissolved in water.
![]()
Object: To show the conduction of current by acids.
Method:
(a) Fix two nails on a cork and place it in a 100 mL capacity beaker. Connect the nails to the terminals of 6V battery, through a bulb and a switch, as shown in figure.
(b) Now take enough dilute HCl in the beaker so that the two nails are immersed in the acid.
Switch on the current. We notice that the bulb starts glowing.
(c) Now repeat the experiment separately with dilute H2SO4. Similar results (as above) are obtained. Thus we can conclude that dilute acids (HCl, H2SO4, etc.) conduct electricity.
(d) Now repeat the experiment separately with glucose solution (C6H12O6) and alcohol (C2H5OH) solution. You will observe that the bulb does not glow in any of the two cases. In other words, glucose and alcohol do not conduct electricity.
Explanations: Glowing of bulb in case of acids indicates that there is a flow of electricity current through the solution. the current as carried through the solution by ions (H+ and Cl– in case of HCl and H+ and SO42– in case of H2SO4). Now all acids contain the cation (positive ion) H+, so this suggests that all acids yield hydrogen ions H+ (aq), in solution, which are responsible for their acidic nature (or properties).
* Uses of Mineral Acids in Industry :
1. Sulphuric acid is used in the manufacture of fertilizers (like ammonium sulphate), paints, dyes, chemicals, plastics, synthesis fibres, detergents, explosives and car batteries.
2. Nitric acid is used for making fertilisers (like ammonium nitrate), explosivs (like TNT : Tri-Nitro Toluene), dyes and plastics.
3. Hydrochloric acid is used for removing oxide film from steel objects (before they are galvanised) and for removing ‘scale’ deposits from inside the boilers.
* PHYSICAL Properties of Bases
The important properties of water soluble bases (or alkali) are given below :
1. Bases have bitter taste.
2. Bases feel soapy to touch.
3. Bases turn red litmus to blue.
4. Bases conduct electricity in solution (They are electrolytes).
* Chemical Properties of Bases
1. Bases react with some metals to form hydrogen gas.
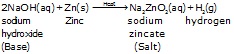
All the metals do not react with bases to form salts and hydrogen.
Object: To show the reaction of bases on metals.
Method: Take a test-tube. Add to it about 2 mL of sodium hydroxide solution and a few pieces of granulated zinc metal. Set up apparatus as shown in figure. Warm the contents of the test-tube and make observations.
Observations:
(a) A gas (hydrogen) is evolved, which on passing through soap solution form soap bubbles filled with H2 gas.
(b) When we burn carefully the soap bubbles filled with H2 gas with a candle, a ‘pop’ sound is boserved.
(c) In the above reaction, hydrogen is displaced from the bases and is evolved as H2 gas.
(d) The metals combine with the remainder part of the bases to form, a compound, called sat. Thus:
Base + Metals ¾® Salt + H2 (g)
e.g ![]()
sodium Zincate
Note: Such reactions are not possible with all metals and this reaction is different from reaction of metals with acids. In this reaction OH– ion gives ZnO22– (zincate ion) and hydrogen gas.
2. Bases react with acids to form salts and water
![]()
sodium sulphuric sodium Water
hydroxide acid sulphate
(Base) (acid) (salt)
when an acid and base combine then the real neutralisation reaction occurs due to the combination to hydrogen ions present in acid and hydroxide ions present in base to form water.
![]()
hydrogen hydroxide Water
ions ions
(From acid) (From base)
3. Bases react with non-metal oxides to form salt and water :
Non - metal oxide + Base ¾¾® Salt + Water
![]()
calcium carbon calcium water
hydroxide dioxide carbonat
(Base) (Non-metal oxide) (salt)
The reactions of non-metal oxides with bases to form salt and water show that non-metal oxides are acidic in nature.
Object: To show the reaction of a base with non-metallic oxides.
Method: Take calcium hydroxide solution (a base) in a test-tube and pass carbon dioxide (a non-metallic oxide) through it. The product is calcium carbonate precipitate (in the form of milkiness) and water. Thus:
![]()
Calcium hydroxide Carbon dioxide
(a base) (an acidic oxide)
Result: From the above activity we can conclude that non-metallic oxides are acidic in nature, since they produce acids with water, e.g.,
![]()
Sulphur dioxide Sulphurous acid
(An acidic oxide)
(i) Sodium hydroxide is used in the manufacture of soap, paper and a synthesis fibre called ‘rayon’.
(ii) Calcium hydroxide ( slaked lime) is used in the manufacture of bleaching powder.
(iii) Magnesium hydroxide is used as an antacid to neutralise excess acid in the stomach and cure indigestion.
What happens when water is mixed with an acid or a base?
Mixing of acid or base in water is called dilution and the acid or the base is said to be diluted.
During dilution, concentration of ions (H3O+ OH–) per unit volume decreases. This process is generally exothermic in nature, which produces heat. Heat produced may be harmful because it may cause the mixture to splash out and cause burns. Moreover the glass container may also break due to excessive local heating. So precautions are recommended during dilution of an acid or bases.
Acidic solutions have excess of hydrogen ions. The basic solutions have excess of hydroxide ions.
In 1909 Sorenson developed a scale (known as pH scale) on which the strength of acid solutions as well as basic solutions could be represented by making use of the hydrogen ion concentration in them.
The pH of a solution is inversely proportional to the concentration of hydrogen ions in it.
The strength of an acid or base in measured on a scale of number called the pH scale. The pH scale has values from 0 to 14.
1. Neutral substances have a pH of exactly 7. The pH of pure water is 7.
A substance having pH 7 will have no effect on litmus or any other common indicator such as methyl orange or phenolphthalein.
2. All the solutions having pH less than 7 are acidic in nature and hence they turn blue litmus to red. They also turn methyl orange indicator red.
3. Bases (or basic solutions) have a pH or more than 7. The more basic a solution is, the higher will be its pH. The higher thepH, the stronger the base (or alkali). All the substances having pH more than 7 are basic in nature (or alkaline in nature) and hence they turn red litmus to blue. They also turn phenolphthalin indicator pink.
Indicator as the name suggests, indicates the nature of particular solution whether acidic, basic or neutral. Apart from this, indicator also represents the change in nature of the solution from acidic to basic & vice-versa, Depending upon the property of the indicator, we have the following two types of acid-base indicators:
(1) Indicators showing different colours in acidic & basic medium.
Examples, Litmus, phenolphthalein and methyl orange.
(2) Indicators giving different odour in acidic and basic medium/olfactory indicators.
Examples, Onion extract, vanilla and clove oil.
Aim: To test acids and bases in the laboratory using colour change acid-base indicators.
Method
(i) Collect the following samples from the science laboratory-hydrochloric acid (HCl), sulphuric acid (H2SO4), nitric acid (HNO3), acetic acid (CH3COOH), sodium hydroxide (NaOH), calcium hydroxide [Ca(OH)2], potassium hydroxide (KOH) , magnesium hydroxide [Mg(OH)2] and ammonium hydroxide (NH4OH).
(ii) Put a drop of each of the above solutions on a watch-glass and test with a drop of the follOwing indicators.
Red litmus solution, Blue litmus solution, Phenolphthalein solution, Methyl orange solution.
Now answer: What change in colour do you observe with each of the above indicators?
Discussion: The changes observed are as follows:
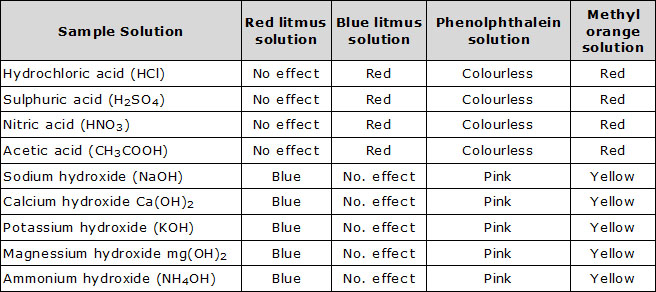
Conclusion:
(i) Acids turn blue litmus red but have no effect on red litmus.
(ii) Bases turns red litmus blue but have no effect on blue litmus.
(iii) Phenolphthalein is colourless in acidic medium and turns pink in basic medium.
(iv) Methyl orange is yellow in basic medium and red in acidic medium.
* Universal Indicators :
Universal indicator is a mixture of many different indicators (or dyes) which gives different colours at different pH values of the entire pH scale.
When an acid or base solution is added to the universal indicator, the indicator produces a new colour. The colour produced by universal
indicator is used to find the pH value of the acid or base solution by matching the colour with the colours on pH colour chart.
Just like litmus, universal indicator can be used either in the form of a solution or in the form of universal indicator paper.
Water will produce a green colour with universal indicator.
² Olfactory indicators :
Those substances whose smell (or odour) changes in acidic or basic solutions are called olfactory indicators. Onion and vanilla extract are olfactory indicators.
Aim: To test acids and bases using olfactory indicators.
Method:
(i) Take some finely chopped onion in a plastic bag along with some strip of dean cloth. Tie up the bag tightly and leave overnight in the fridge.
The cloth strips can now be used to test for acids and bases.
(ii) Take two of these cloth strips and check their odour.
(iii) Keep them on a clean surface and put a few drops of dilute HCl solution on one strip and a few drops of dilute NaOH solution on the other.
(iv) Rinse both cloth strips with water and again check their odour.
(v) Note your observations.
(vi) Now take some dilute vanilla essence and clove oil and check their odour.
(vii) Take some dilute HCl solution in one test tube and dilute NaOH solution in another. Add a few drops of dilute vanilla essence to both test tubes and shake well.
Check the odour once again and record change in odour, if any.
(viii) Similarty, test the change in the odour of clove oil with dilute HCl and dilute NaOH solutions and record your observation.
Discussion: On the basis of your observations, report which of the following vanilla, onion and clove oil can be best used as an olfactory indicator.
Conclusion: A substance which has an odour in acidic medium and different odour in basic medium can be used as an acid base indicator (caped olfactory indicator).

1. pH in our Digestive System :
Our stomach produces hydrochloric acid helps in digesting our food. The excess acid in the stomach causes indigestion which produces
pain and irritation. Antacids are a group of mild bases which have no toxic effects on the body, react with excess acid in the stomach and neutralise it.
The two common antacids used for curing indigestion due to acidity are : Magnesium hydroxide (Milk of Magnesia) and Sodium hydrogen carbonate (Baking soda).
2. pH change as the cause of Tooth decay :
The bacteria present in our mouth break down the sugar to form acids. This acid lowers the pH in the mouth (making decay) is to clean
the mouth thoroughly after eating food.
Tooth enamel is made up of calcium phosphate, the hardest substance in our body and it does not dissolve in water. Tooth starts decaying,
when pH of the mouth is less than 5.5, due to corrosion of calcium phosphate. Bacteria present in the mouth produce acid by the degradation
of carbohydrates (like sugar) and food particles still remaining in the mouth after eating. The best method of preventing tooth decay is to clean
the mouth after eating and avoid eating sugar foods (such as sweets, toffees etc.). Most common tooth pastes are basic in nature so by using
these for cleaning the teeth, the excess acid gets neutralized thereby pH becomesgreater than 5.5 and tooth decay is prevented.
3. Plants and Animals are Sensitive to pH Changes :
Soil pH and Plant Growth : Most of the plants grow best when the pH of the soil is close to 7. If the soil is too acidic (having low pH),
then it is treated with materials like quicklime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate).
If the soil is too alkaline then its alkalinity can be reduced by adding decaying organic matter (manure or compost) which contains acidic materials.
In plants and animals: Living organisms can survive only in a narrow range of pH values. eg.g, our body can work within 7.0 to 7.5 pH range.
When pH of rain water is below 5.6 (due to absorption of acidic gases like CO2, SO2, NO2 etc. present n the atmosphere), it is known as acid rain,
which on flowing into the rivers lowers the pH of the river water, thereby survival of aquatic life in such rivers becomes difficult.
4. Self Defence by Animals and Plants Through Chemical Warfare :
Many animals and plants protect themselves from their enemies by injecting painful and irritating acids and bases into their skin.
For example when a honey bee stings a person, it injects an acidic liquid into the skin which causes immense pain and irritation.
An ant’s sting injects methanoic acid (HCOOH) into the skin of a person causing burning pain.
When a person happens to touch the leaves of a nettle plant accidently, the stinging hair of nettle leaves inject methanoic
acid into the skin of the person causing burning pain.
pH Value of Some Common Substances
Salts are the ionic compounds consisting of two parts, one part carrying a positive charge called positive ion or cation and
the other part carrying a negative charge called a negative ion or anion.
Some common salts
1. Sodium chloride (NaCl) 2. Sodium sulphate (Na2SO4)
3. Potassium nitrate (KNO3) 4. Copper sulphate (CuSO4)
5. Zinc sulphate (ZnSO4) 6. Calcium carbonate (CaCO3)
7. Calcium chloride (CaCl2) 8. Aluminium sulphate [Al2(SO4)3]
Salts are formed when acids reacts with bases
Types of Salts
(i) The salts of ‘hydrochloric acid’ are called ‘chlorides’.
(ii) The salts of ‘sulphuric acid’ are called ‘sulphates’,
(iii) The salts of ‘nitric acid’ are called ‘nitrates’.
(iv) The salts of ‘carbonic acid‘ are called ‘carbonates’.
(v) The salts of ‘acetic acid’ are called ‘acetates’ and so on.

Aim: To find the pH of the given salt solutions.
Method:
(i) Collect the following salt samples:
(a) Sodium chloride (b) Potassium nitrate
(c) Aluminium chloride (d) Zinc sulphate
(e) Copper sulphate (f) Sodium acetate
(g) Sodium carbonate (h) Sodium hydrogen carbonate
(ii) Check their solubility in water (use distilled water only).
(iii) Check the action of these solutions on litmus and find the pH using a pH paper.
(iv) Report your observations in a tabular form.
Now answer
(i) Which of the salts are acidic, basic or neutral?
(ii) Identify the acid or base used to form the salt.
1. Sodium Chloride (Common salt/table salt)
We know that hydrochloric acid and sodium hydroxide combine with each other to form sodium chloride (NaCl) which in
common language is also known as common salt. This is the salt which you sprinkle on your
salads and use in your kitchens. Common salt is an ionic compound of sodium and chlorine
(Na+Cl–)n.
The main source of common salt (sodium chloride) is the sea water. Sea water contains about 3.5% of soluble salts,
the most common of which is sodium chloride (2.7 to 2.9%). Saline water of inland lakes, such as Sambhar lake in Rajasthan
is also a good source of common salt. Sodium chloride is also found as rock salt. Beds of
rock salt were formed when lakes/seas dried up in past. Common salt act as raw material for making various materials of
daily use. Let us discuss some of them.
2. Sodium hydroxide (NaOH) (chlor alkali processes): When electricity is passed through an acidulated aqueous solution of brine
(or sodium chloride) the salt undergoes decomposition to produce sodium metal at cathode and chlorine gas at anode.
The sodium metal then reacts with water to form sodium hydroxide and evolves hydrogen gas at cathode.
![]()
At cathode:
![]()
![]()
3. Bleaching powder (CaOCl2): The chlorine gas (obtained during the electrolysis of brine) is passed through dry slaked lime
[Ca(OH)2], then bleaching powers (CaOC2) is obtained.
![]()
Dry slaked lime Calcium oxychloride
(Bleaching powder)
Uses:
(i) For disinfecting (or sterlizing) water (making water free of germs).
(ii) As a germicide and deodorant in medicine.
(iii) For rendering wool unshrinkable.
(iv) For laboratory preparation of chlorine and oxygen.
(v) In the manufacture of chloroform.
(vi) For bleaching, cotton and linen in the textile industry and wood pulp in paper factories and for bleaching wahed clothes in laundary.
4. Baking soda (or sodium hydrogen carbonate, NaHCO3): Is prepared by passing carbon dioxide gas through a cold saturated solution of sodium carbonate.
![]()
sodium carbonate Sodium hydrogen carbonate
Due to its low solubility in cold water, it separates out as white crystals. It is also the primary product of the Solvay process.
Uses:
(i) As an ingredient in antacid medicines, since it is alkaline in nature and neutralizes excess acid in the stomach.
(ii) In cooking food in the form of baking powers, which contains sodium hydrogen carbonate and an acid like tartaric acid
(or salt of tartaric acid such as potassium hydrogen tartarate). When baking powder is either dissolved in water or heated,
its constituent sodium hydrogen carbonate undrgoes decomposition to liberate carbon dioxide, which causes the bread and
cake to rise. Tartaric acid present in the baking powder neutralizes sodium carbonate.
![]()
If tartaric acid (or its acidic salt) is not present in the baking powder, then the cake/bread will taste bitter, due to the presence of sidum carbonate.
(iii) In soda-acid fire extinguisher, which contains a solution of sodium hydrogen carbonate surrounding a glass bottle containing sulphuric acid.
In case of fire, the knob (provided at the top of extinguisher) is pressed,
thereby bottle breaks and the two solutions come in contract, thereby liberating carbon dioxide gas.
![]()
The liberated carbon dioxide forces a stream of effervescing liquid on the fire. In this way, carbon dioxide surrounds the combustible substances
and cut-off the supply of air, thereby it assits to put out the fire.
(iv) In effervescent cold drinks and fruit salts (e.g. Eno).
5. Sodium carbonate or washing soda, Na2CO3. 10 H2O: Is manufactured by solvay process.
The raw materials employed are sodium chloride (NaCl), limestone (CaCO3) and ammonia (NH3). Actually, most of the ammonia
is recovered in the process itself. In this process:
Step I: A cold and concentrated solution of sodium chloride (called brine) is saturated with ammonia to get ammoniacal brine.
Step II: The ammoniacal brine is fed from the top of carbonating tower, which is provided with perforated plates. As the ammoniacal
brine trickles down this tower, upcoming carbon dioxide reacts with ammoniacal brine to form insoluble precipitate of sodium hydrogen
carbonate (or sodium bicarbonate, NaHCO3). Thus:
The sodium hydrogen carbonate is filtered; while ammonium chloride is recycled in step I to prepare ammonia.
Step III: Sodium hydrogen carbonate precipitate is heated to get soda ash (sodium carbonate).
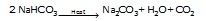
Soda ash
(Sodium carbonate)
The CO2 is recycled to step (II).
Step IV: The soda ash (Na2CO3) so-obtained is dissolved in water and crystallized to get washing soda.
![]()
Soda ash Washing soda
It dissolves in water to give alkaline solution. So its aqueous solution turns red litmus blue.
Uses:
(i) For washing purposes in the laundry.
(ii) For softening hard water.
(iii) As a laboratory regent.
(iv) For manufacturing fusion mixture (Na2CO3 + K2CO3), borax, sodium phosphate, glass, soap etc.
6. Plaster of Paris, CaSO4. ½H2O: Is prepared by heating gypsum (CaSO4 . 2H2O) at 373 K in a kiln.
![]()
Gypsum Plaster of Paris
It may be pointed that heating should be carried our carefully at controlled temperature of 373 K. If temperature of heating
exceeds 373 K, there is every possibility of formation of anhydrous calcium sulphate (CaSO4), which possesses no
property of Plaster of Paris.
Properties:
(i) A white powder, which abosrbs water with evolution of heat.
(ii) Plaster of Paris (CaSO4 . ½H2O) possesses the property of “setting up” on mixing with water. During this process, heat is
evolved and the material quickly sets with a little expansion. The setting of Plaster of Paris is due to its hydration to gypsum.
![]()
Plaster of Paris Gypsum
Uses:
(i) For making casts for statues, metals etc.
(ii) In orthopedic to maintain bone joints in fixed position.
(iii) In making wall-boards.
(iv) As a fire-proofing material.
(v) For making smooth the surfaces of walls, ceilings, etc. This process is known as POP.
(vi) For making ornate designs on walls, ceilings etc.
(vii) For making toys and decorative materials.
(viii) In laboratories and industries for sealing the air gap in apparatus/equipment so as to make it air tight.
Q. There will not be any change in the colour of the pH paper because NaCl is a neutral salt.
Are the crystals of salts really dry?
Crystals of some salts contain certain amount of associated water.
The water associated with the crystal (or molecule) of any salt is called water of crystallisation.
The salt containing water of crystallisation are called hydrated salts.
Aim: To test the presence of water of crystallisation in copper sulphate crystals.
Method:
(i) Heat a few crystals of copper sulphate in a dry boiHng tube.
(ii) Note the colour of copper sulphate after heating.
(iii) Observe the water droplets in the boiling tube.
(iv) Add 2-3 drops of water on the sample of copper suiphate obtained after heating.
Now Answer
(i) What do you observe on heating blue copper sulphate crystals?
(ii) Is the blue colour of copper sulphate restored on adding water?
Discussion
Blue coloured copper sulphate crystals heating leave behind white anhydrous copper sulphate and water droplets are seen in
the upper cooler parts of the boiling tube. On adding 2-3 drops of water to the white residue. blue colour reappears.
Conclusion
Copper sulphate crystals on heating lose water to form white anhydrous copper sulphate which
combines with water to form blue coloured copper sulphate crystals.
Why plaster of Paris cannot be employed ad drying agent?
Anhydrous calcium sulphate (CaSO4) takes up water readily, forming hydrated salt, gypsum.
![]()
Gypsum
On the other hand, plaster of Paris sets on addition of water. So it cannot be used as a drying agent.
Water of Crystallisation
It is the fixed number of water molecules present in one formula unit of a crystalline salt, e.g..,
![]()
Ex.1 Give two physical characteristics each of acids and bases ?
Sol. Acids are
(i) Sour in taste (ii) Change the colour of blue litmus to red.
Base are
(iii) Bitter in taste (iv) Change the colour of red litmus to blue.
Ex.2 What are indicators ? Name four acid - base indicators and mention their colour change ?
Sol. Indicators are chemical substances which give different colours in acidic or basic solutions :
(i) Methyl Orange gives orange colour with acid solution and yellow colour with base solution.
(ii) Phenolphthalein is colourless in acid solution while it turns into pink colour in base solution.
(iii) Litmus solution turns red in acid solution and blue in base solution.
(iv) Bromothymol blue is blue in base solution and is yellow in acid solution.
Ex.3 What are antacids ?
Sol. Antacids are mild alkalies and contain sodium hydrogen carbonate. These are used for getting relief from acidity
and indigestion and sometimes, even headache. When taken orally, it reacts with hydrochloric acid
present in the stomach and reduces its strength by consuming some of it. For example, Milk of Magnesia (MgO).
Ex.4 What are olfactory indicators ?
Sol. Olfactory indicators are substances which have different odour in acid and base solutions, For example vanilla essence and clove oil.
Ex.5 What is a neutralization reaction ? Give some example.
Sol. When the effect of a base is nullified by an acid and vice versa, it is called neutralization reaction. In general, a neutralization reaction is written as :
Base + Acid ¾¾® Salt + Water
Examples :
(i) Aqueous solution of base, NaOH is neutralized by aqueous hydrochloric acid
NaOH(aq) + HCl(aq) ¾¾® NaCl(aq) + H2O
(ii) Aqueous solution of sulphuic acid is neutralized by aqueous solution of sodium hydroxide.
H2SO4(aq) + 2NaOH(aq) ¾¾® Na2SO4(aq) + 2H2O
Ex.6 When hydrochloric acid is added to marble pieces, a gas (A) is evolved. On passing gas A through lime water, a white precipitate of
(B) is formed. When excess of A is passed B dissolves due to the formation of soluble C. Identify A, B and C. Explain the reaction.
Sol. A. Carbon dioxide
B. Calcium carbonate
C. Calcium hydrogen carbonate
Chemical reaction
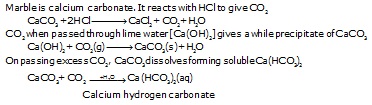
Ex.7 Name four acids and bases. Write their formulate.
Sol. Acids Bases
(i) Hydrocholoric acid - HCl (i) Sodium hydroxide - NaOH
(ii) Sulphuric acid - H2SO4 (ii) Calcium hydroxide - Ca(OH)2
(iii) Nitric acid - HNO3 (iii) Ammonium hydroxide - NH4OH
(iv) Acetic acid - CH3COOH (iv) Magnesium hydroxide - Mg(OH)2
Ex.8 Mention important characteristics of acids.
Sol. Characteristics of acids :
(i) They are sour in taste.
(ii) They turn blue litmus to red.
(iii) Acids react with metals to envolve hydrogen gas.
![]()
(iv) Acids react with bases to form salt and water. This is called neutralisation.
![]()
(v) Acids react with basic oxides to form salt and water.
![]()
(vi) Acid react with carbonate to form salt, water and CO2
![]()
Ex.9 A solution turns red litmus paper blue. What does this indicate about the chemical nature of the solution ?
Sol. The changing colour of litmus from blue to red is a characteristics of the substances called bases or alkalis.
Metal hydroxide like potassium hydroxide change the colour of red litmus to blue litmus.
Ex.10 What are bases ? Give the characteristics of bases.
Sol. Bases are the hydroxide of metals which give hydroxide ion after dissociation in aequeous solution.
Characteristic of bases
(i) They are bitter in taste.
(ii) They change red litmus to blue.
(iii) They react with acids to form salt and water.
(iv) Bases are soluble in water and are known as alkalies.
Ex.11 How do you measure the strength of an acid or a base ?
Sol. The strength of an acid or a base depends on the number of H+ ions or OH– ions produced respectively by its
given amount. If we take one molar concentration (1 mole dissolved in 1 litre) of hydrochloric acid and
acetic acid, then the acid which gives rise to more of H+ ions is a stronger acid and the one that gives less H+
ions is a weaker acid. In this case, it is found that hydrocholoric acid is a strong acid. Similarly one can find
whether it is a strong base or a weak base. (Here number of OH– ions is counted).
Ex.12 1 mole per litre of (A) has pH equal to 13 and 1 mole per litre of (B) has pH equal to 11.
Which is stronger ? Whether these are bases or acids ?
Sol. Both A and B are bases since their pH values are more than 7. Since both have equal concentration and the pH of A is more than that of B, A is a stronger base than B.
Ex.13 A group of students measured the pH of some substance they found in their home. Their results are given in the table below :
Substance pH
Apples 3.0
Black coffee 8.5
Lemon juice 2.5
Milk 6.5
Sugar 7.0
Toothpaste 9.0
Vinegar 3.0
Washing soda 11.5
(i) Which solution is the most acidic ?
(ii) Which solution is the most basic ?
(iii) Which substance is the neutral
Sol. (i) Lemon juice (ii) Washing soda (iii) Sugar
Ex.14 What is acid rain. How does it affect our aquatic life ?
Sol. When the pH of rain water is less than 5.6, it is called acid rain. When acid rain flows in to the rivers, it lowers the pH of the river water.
Since our body works within a narrow pH range close to 7, the survival of aquatic life in river waters mixed with rain water becomes difficult.
Ex.15 The tanks in which milk is stored for retail selling are cleaned with sodium hydroxide solution everytime fresh milk is filled in them.
Give the reason for this practice.
Sol. Milk contains lactic acid and its effect remains in the empty tank. The acidic effect in the tank can be neutralized by cleaning with it with a
solution of a base, e.g., NaOH. It changes the acid into salt which then gets removed from the tank by cleaning with water.
Q.1 You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and
a basic solution, respectively. If you are given only red litmus paper. how will you identify the contents of each test tube?
Ans. Step 1: Let us mark the three test tubes as A, B and C.
Step 2: A drop of the solution in test tube A is put on the red litmus paper. Same is repeated with solution Band C. If either of
them changes colour to blue, then it is basic. Therefore, out of three, one is eliminated.
Step 3: Out of the remaining two, anyone can be acidic or neutral. Now a drop of basic solution is mixed with a drop of each of the
remaining two solutions separately and then a drop of each solution is put on the red litmus paper. If the colour of red litmus turns blue,
then that solution is neutral and if there is no cha.nge in colour, then that solution is acidic. This is because acidic and basic solutions
neutralise each other. Hence, we can distinguish between the three types of solutions.
Q.2 Why should curd and sour substances not be kept in brass and copper vessels?
Ans. Curd and other sour substances contain acids. Therefore, when they are kept in brass and copper vessels, the metal reacts with the
acid to liberate hydrogen gas and harmful products, thereby spoiling the food.
Q.3 Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Ans. Hydrogen gas is usually liberated when an acid reacts with a metal.
Example : Procedure :
Step 1 : Take few pieces of zinc granules and add 5 ml of dilute H2SO4 .
Step 2 : Shake it and pass the gas produced into a soap solution. The bubbles are formed in the soap solution.
These soap bubbles contain hydrogen gas. Test for hydrogen gas: The evolved hydrogen gas can be
tested by bringing a burning candle near the soap bubbles. Hydrogen gas burns with a pop sound.
Q.4 Metal compound A reacts with dilute hydrochloric acid to produce effervescence. The gas evolved extinguishes a burning candle.
Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Ans. ![]()
Q.5 Why do HCl, HNO3, etc., show acidic character in aqueous solution while solutions of compounds like alcohol and glucose do not show acidic character?
Ans. HCl and HNO3 dissociate in the presence of water to form hydrogen or hydronium ions. Although aqueous solutions of glucose and alcohol contain hydrogen,
these cannot dissociate in water to form hydrogen or hydronium ions. Hence, they do not show acidic character.
Q.6 Why does an aqueous solution of an acid conduct electricity?
Ans. Acids dissociate in aqueous solutions to form ions. These ions are responsible for conduction of electricity.
Q.7 Why does dry HCl gas not change the colour of the dry litmus paper?
Ans. Dry HCl gas does not change the colour of the dry litmus paper because it does not contain H+ ions.
Q.8 While diluting an acid, why is it recommended that the acid should be added to water and not water to the acid?
Ans. It is recommended that the acid should be added to water and not water to the add because the process of dissolving an
acid in water is exothermic.If water is added to acid. since large amount of acid is present, a large amount of heat is generated.
therefore, the mixture splasnes out and causes burns.
Q.9 How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted?
Ans. When an acid is diluted, the concentration of hydronium ions (H3O+) per unit volume decreases.
This means that the strength of the acid decreases.
Q.10 How is the concentration of hydroxide ions (OH–) affected when excess base is dissolved in a solution of sodium hydroXide ?
Ans. The concentration of hydroxide ions (OH–) would increase when excess base is dissolved in a solution of sodium hydroxide.
Q.11 You have two solutions, A and B. The pH of solution A is 6 and pH of solution B is 8. Which solution has more hydrogen ion
concentration? Which of this is acidic and which one is basic ?
Ans. A pH value of less than 7 indicates an acidic solution, while greater than 7 indicates a basic solution. Therefore, the solution
with pH 6 is acidic and has more hydrogen ion concentration than the solution of pH 8 which is basic.
Q.12 What effect does the concentration of H+(aq) ions have on the nature of then solution ?
Ans. If the concentration of H+ ions is increased, the solution becomes more acidic. If the concentration of H+ ions is decreased,
the solution becomes less acidic or more basic.
Q.13 Do basic solutions also have H+(aq) ions? If yes, then why are these basic ?
Ans. Yes, a basic solution also has H+(aq) ions. However, their concentration is less as compared to the concentration of OH-(aq)
ions which makes the solution basic.
Q.14 Under what soil condition do you think a farmer would treat the soil of his fields with quick lime
(calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate) ?
Ans. If the soil is acidic and improper for cultivation, then to neutralise the acidity of the soil, the farmer would treat the soil
with quick lime or slaked lime or chalk.
Q.15 What is the common name of the compound CaOCl2.
Ans. The common name of the compound CaOCl2 is bleaching powder.
Q.16 Name the substance which on treatment with chlorine yields bleaching powder?
Ans. Calcium hydroxide [Ca(OH)2], on treatment with chlorine, yields bleaching powder.
Q.17 Name the sodium compound which is used for softening hard water.
Ans. Washing soda (Na2CO3.10 H2O) is used for softening hard water.
Q.18 What will happen if a solution of sodium hydrogen carbonate is heated? Give the equation of the reaction involved.
Ans. When a solution of sodium hydrogencarbonate is heated, sodium carbonate and water are formed With the evolution of carbon dioxide gas.
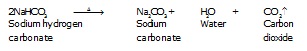
Q.19 Write an equation to show the reaction between Plaster of Paris and water.
Ans. The chemical equation for the reaction of Plaster of Paris and water can be represented as:
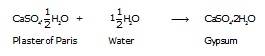
Q.20 A solution turns red litmus blue, its pH is likely to be
(1) 1 (2) 4 (3) 5 (4) 10
Ans. (4) 10
Q.21 A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains
(1) NaCl (2) HCl (3) LiCl (4) KCl
Ans. (2) HCl
Q.22 10 mL of a solution of NaOH is found to be completely neutralised by 8mL of a given solution of HCl.
If we take 20 mL of the same solution of NaOH, the amount of HCl solution (the same solution as before)
required to neutralise it will be
(1) 4 mL (2) 8mL (3) 12 mL (4) 16 mL
Ans. (4) 16 mL
Q.23 Which one of the follwing types of medicines is used for treating indigestion ?
(1) Antibiotic (2) AnalgesiC (3) Antacid (4) Antiseptic
Ans. (3) Antacid
Q.24 Write word equations and then balanced equations for the reaction taking place when
(1) Dilute sulphuric acid reacts with zinc granules.
(2) Dilute hydrochloric add reacts with magnesium ribbon.
(3) Dilute sulphuric acid reacts with aluminium powder.
(4) Dilute hydrochloric acid reacts with iron filings.
Ans. (1) Sulphuric acid + zinc ¾® Zinc sulphate + Hydrogen

Q.25 Compounds such as alcohols and glucose also contain hydrogen but are not categorized as acids. Describe an activity to prove it.
Ans. Acitivity : Procedure :
Step 1: Two nails are fitted on a cork and are kept it in a 100 mL beaker.
Step 2: The nails are then connected to the two terminals of a 6-volt battery through a bulb and a switch.
Step 3: Some dilute HCl is poured in the beaker and the current is switched on.
Step 4: The same experiment is then performed with glucose solution and alcohol solution.
Observations: glows in the HCl solution and does not glow In the glucose solution and alcohol solution. Result: HCl dissociates into H+
(aq) and C!iaq) ions. These ions conduct electricity in the solution which results in the glowing of the bulb. On the other hand, the glucose
solution and alcohol solution does not dissociate into ions. Therefore, it does not conduct electricity.
Conclusion: From this activity, it can be concluded that all acids contain hydrogen but not all compounds containing hydrogen are acids.
That is why, though alcohols and glucose contain hydrogen, they are not categorised as acids.
Q.26 Why does distilled water not conduct electricity, whereas rain water does?
Ans. Distilled water is a pure form of water and is devoid of any ionic species. Therefore, it does not conduct electricity. Rain water, being
an impure form of water, contains many ionic species such as acids and therefore it conducts electricity.
Q.27 Why do acids not show acidic behaviour in the absence of water?
Ans. Acids do not show acidic behaviour in the absence of water because there are no hydrogen ions available for conduction of electricity.
Acids dissociate in the presence of water to give free hydrogen ions. It is the hydrogen ions that are responsible for the acidic behaviour.
Q.28 Five solutions A, B, C, D and E when tested with universal indicator showed pH as 4, 1, 11, 7 and 9, respectively. Which solution is
(a) neutral ? (b) strongly alkaline ? (c) strongly acidic?
(d) weakly acidic ? (e) weakly alkaline ?
Arrange the pH in increasing order of hydrogenion concentration.
Ans. (a) Neutral - Solution D with pH 7
(b) Strongly alkaline - Solution C with pH 11
(c) Strongly acidic - Solution B with pH 1
(d) Weakly acidic - Solution A with pH 4
(e) Weakly alkaline - Solution E with pH 9
The pH can be arranged in the increasing order of the concentration of hydrogen ions as:
11 < 9 < 7 < 4 < 1.
Q.29 Equal lengths of magnesium ribbons are taken in test tubes A and B. Hydrochloric acid (HCl) is added to test tube A.
while acetic acid (CH3COOH) is added to test tube B. In which test tube will the fizzing occur more vigorously and why?
Ans. The fizzing will occur strongly in test tube A, in which hydrochlOric acid (HCl) is added. This is because HCl is a stronger
acid than CH3COOH and therefore produces hydrogen gas at a faster speed due to which fizzing occurs.
Q.30 Fresh milk has a pH of 6. How do you think the pH will change as it turns into curd? Explain your answer.
Ans. The pH of milk is 6. As it changes to curd, the pH will reduce because curd is acidic in nature. The acids present in it decrease the pH.
Q.31 A milkman adds a very small amount of baking soda to fresh milk.
(a) Why does he shift the pH of the fresh milk from 6 to slightly alkaline?
(b) Why does this milk take a long time to set as curd?
Ans. (a) The milkman shifts the pH of the fresh milk from 6 to slightly alkaline because in alkaline condition, milk does not set as curd easily.
(b) Since this milk is slightly basic than usual milk, acids produced to set the curd are neutralised by the base. Therefore, it takes a longer time for the curd to set.
Q.32 Plaster of Pans should be stored in a mOisture-proof container. Explain why?
Ans. Plaster of Paris should be stored in a moisture-proof container because Plaster of Paris. a powdery mass,
absorbs water (moisture) to form a hard solid known as gypsum.
Q.33 What is a neutralisation reaction ? Give two examples.
Ans. A reaction in which an acid and base react with each other to give a salt and water is termed as neutralisation reaction.
In this reaction, energy is evolved in the form of heat.
For example:
![]()
(ii) During indigestion (casued due to the production of excess of hydrochloric acid in the stomach), we administer an antacid
(generally milk of magnesia, Mg(OH)2 which is basic in nature). The antacid neutralises the excess of acids and thus gives relief from indigestion.
![]()
Q.34 Give two important uses of washing soda and baking soda.
Ans. Two important uses of washing soda and baking soda are as follows:
(1) Washing soda :
(a) It is used in glass, soap, and paper industries.
(b) It is used to remove permanent hardness of water.
(2) Baking soda:
(a) It is used as baking powder. Baking powder is a mixture of baking soda and a mild acid known as tartaric acid. When it is heated or mixed in water,
it releases CO2 gas that makes bread or cake fluffy.
(b) It is used in soda-acid fire extinguishers.
Q.1 What happens when
(a) Baking soda is heated
(b) Blue colour copper sulphate crystals heated
(c) Water is added to lime?
Q.2 A white powder which sets hard on adding water is also used in hospitals. Name this powder. How is it prepared? Write the chemical reaction involved in its preparation
Q.3 Write balanced chemical equations for the following;
(a) Calcium carbonate reacts with hydrochloric acid
(b) dilute sulphuric acid reacts with Zinc granites
(c) Calcium oxychloride reacts with hydrochloric acid
Q.4 Name the ions present in the following salts. Name the acid and base from which they can be obtained. Magnesium sulphate, sodium carbonate, potassium chloride.
Q.5 Give three ways in which salts can be prepared,
Q.6 Give one example for each of the following acids salts chloride salts, carbonate salts, sulphate salts.
Q.7 Name the acid present in the following.
Vinegar; Lemon, Tomoto, Tamarind, orange, curd.
Q.8 Acid when react with metals release hydrogen gas but there is one acid which when reacts with metals and does not release hydrogen except for two metals. Prove this statement.
Q.9 When carbon dioxide is bubbled into limewater, a white cloud appears.
(i) Write an equation to show the reactionbetween limewater and water.
(ii) Name and write the formula of the product.
(iii) What is the chemical name for limewater.
(iv) Write the equation for the chemical reaction between lime water, water and CO2
Q.10 Why does common salt become moist in rainy season.
Or
Ordinary common salt contains another substance which is deliquescent. Name the substance and write its formula.
Q.11 An alkali is an important base used for the laboratory work. Name the base and state how it can be prepared from common salt ?
Q.12 Give the different commercial forms of sodium carbonate.
Q.13 What is efflorescence ?
Q.1 A solution turns red litmus blue, its pH is likely to be–
(A) 1 (B) 4 (C) 5 (D) 10
Q.2 A solution reacts with crushed egg-shells to give a gas that turns lime-water milky. The solution contains–
(A) NaCl (B) HCl (C) LiCl (D) KCl
Q.3 Which one of the following types of medicines is used for treatment indigestion–
(A) Antibiotic (B) Analgesic (C) Antacid (D) Antiseptic
Q.4 According to Arrhenius acid gives –
(A) H+ in water (B) OH– in water (C) Both (A) & (B) (D) OH– in acid medium
Q.5 Milk of magnesia is an –
(A) Acid (B) Antacid (C) Alkali (D) Rock salt
Q.6 Noble metals are dissolved in –
(A) Conc. HNO3 (B) Conc. HCl (C) Conc. H2SO4 (D) Aqua-regia
Q.7 Which of the following is not a strong acid?
(A) H2SO4 (B) CH3COOH (C) HNO3 (D) HCl
Q.8 Soda ash is –
(A) Na2CO3·H2O (B) Na2CO3 (C) NaOH (D) NaHCO2
Q.9 Which of the following method is not used in preparing a base?
(A) Burning of metal in air.
(B) Adding water to a metal oxide.
(C) Reaction between an acid and base.
(D) Heating metal carbonates.
Q.10 Fats + NaOH ¾® ........+ Glycerol. One of the product formed in this reacton is –
(A) Soap (B) Cloth (C) Paper (D) Wood
Q.11 Potash alum is a ?
(A) Simple salt (B) Complex salt (C) Acid salt (D) Double salt
Q.12 NaHCO3 represent the formula of which one of the following ?
(A) Sodium carbonate
(B) Baking soda
(C) Sodium acetate
(D) Washing soda
Q.13 _____ acid is responsible for the ‘fizz’ in soft drinks. [NTSE]
(A) Oxalic (B) Acetic (C) Citric (D) Carbonic
Q.14 A base can be prepared by the reaction between ________. [NTSE]
(A) an active non-metal and water
(B) a gas and water
(C) a sulphide and water
(D) an active metal and water
Q.15 Identify the base in the given reaction:
HI + H2O ¾® H3O+ + I–. [NTSE]
(A) HI (B) H2O (C) H3O– (D) I–
Q.16 In the following reaction, identify the products Na2CO3(aq) + 2HCl (aq) ¾® ____ [NTSE]
(A) NaCl + H2O
(B) H2O + CO2
(C) Na2CO3 + CO2 + H2O
(D) NaCl + CO2 + H2O
Q.17 In the following acid-base neutralisation reaction: [NTSE]
CH3COOH + NaOH ¾® CH3COONa + H2O which term best describes the formation of sodium acetate (CH3COONa)?
(A) Salt (B) Acid (C) Base (D) Neutralisation agent
Q.18 Which of the following is not a mixed salt? [NTSE]
(A) KCaPO4 (B) Ca(OCl)Cl (C) NaKCO3 (D) KCl
Q.19 The correct order of Lewis acid character is ____. [NTSE]
(A) BH3 < BCl3 < BBr2 < BI3
(B) BCl3 < BF3 < BBr3 < BI3
(C) BBr3 < BI3 < BCl3 < BF3
(D) BF3 > BCl3 > BBr3 > BI3
Q.20 Soda water has a pH of _______. [NTSE]
(A) less than 7
(B) more than 7
(C) equal to 7
(D) cannot say
Q.21 The fizzing out of gas on opening a soda water bottle is because of _____. [NTSE]
(A) increase in the pressure and splitting of gas molecules
(B) decrease in pressure and increase in solubility of gas
(C) decrease in pressure and solubility of gas
(D) increase in pressure of gas leading to evolution of new product
Q.22 In aluminothermite process, aluminium acts as _____. [NTSE]
(A) an oxidising agent
(B) a flux
(C) a reducing agent
(D) a solder
Q.23 Which of the following reactions occur at cathode? [NTSE]
(A) 2OH– ¾® H2O + O + 2e–
(B) Fe2+ ¾® Fe3+ + e–
(C) Ag ¾® Ag+ + e–
(D) Cu2+ + 2e– ¾® Cu
Q.24 A solution of sodium sulphate in water is electrolysed using inert electrodes. The products at cathodes and anode, respectively, are ______. [NTSE]
(A) H2 and O2
(B) O2 and H2
(C) O2 and Na
(D) O2 and SO2
Q.25 The stronger the oxidising agent, greater is the _______. [NTSE]
(A) reduction potential
(B) oxidation potential
(C) ionic behaviours
(D) none of the above
Q.26 In the following reaction, identify that salt formed: [NTSE]
2NH4OH(aq) + H2SO4(aq) ¾® _____ + 2H2O(l)
(A) NH4NO3
(B) (NH4)2SO4
(C) (NH4)PO4
(D) (NH4)2S
Q.27 Which of the following salts does not contain water of crystallisation? [NTSE]
(A) Blue vitriol
(B) Baking soda
(C) Washing soda
(D) Gypsum
Q.28 Which of the following gives the correct increasing order of acidic strength? [NTSE]
(A) Water < Acetic acid < Hydrochloric acid
(B) Water < Hydrochloric acid < Acetic acid
(C) Acetic acid < Water < Hydrochloric acid
(D) Hydrochloric acid < Water < Acetic acid
Q.29 If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done? [NTSE]
(A) Wash the hand with saline solution
(B) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogencarbonate
(C) After washing with plenty of water apply solution of sdoium hydroxide on the hand
(D) Neutralise the acid with a strong alkali
Q.30 Sodium hydrogencarbonate when added to acetic acid evolves a gas. Which of the following statements are true about the gas evolved? [NTSE]
(i) It turns lime water milky
(ii) It extinguishes a burning splinter
(iii) It dissolves in a solution of sodium hydroxide
(iv) It has a pungent odour
(A) (i) and (ii)
(B) (i), (ii) and (iii)
(C) (ii), (iii) and (iv)
(D) (i) and (iv)
Q.31 Common salt besides being used in kitchen can also be used as the raw material for making
(i) washing soda (ii) bleaching powder
(iii) baking soda (iv) slaked lime [NTSE]
(A) (i) and (ii)
(B) (i), (ii) and (iv)
(C) (i) and (iii)
(D) (i), (iii) and (iv)
Q.32 One of the constituents of baking powder is sodium hydrogencarbonate, the other constituent is [NTSE]
(A) hydrochloric acid
(B) tartaric acid
(C) acetic acid
(D) sulphuric acid
Q.33 Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the
pH of the resulting solution is checked with a pH paper. What would be the colour obtained? [NTSE]
(A) Red
(B) Yellow
(C) Yellowish green
(D) Blue
Q.34 Which of the following is(are) true when HCl(g) is passed through water? [NTSE]
(A) It does not ionise in the solution as it is covalent compound.
(B) It ionises in the solution
(C) It gives both hydrogen and hydroxyl ion in the solution
(D) It forms hydronium ion in the solution due to the combination
Q.35 Which of the following are present in a dilute aqueous solution of hydrochloric acid?[NTSE]
(A) H3O+ + Cl–
(B) H3O+ + OH–
(C) Cl– + OH–
(D) unionised HCl
Q.36 Identify the correct representation of reaction occuring during chlorakali process [NTSE]
(A) 2NaCl(l) + 2H2O(l) ¾® 2NaOH(l) +Cl2(g) + H2(g)
(B) 2NaCl(aq) + 2H2O(aq) ¾® 2NaOH(aq) + Cl2(g) + H2(g)
(C) 2NaCl(aq) + 2H2O(l) ¾® 2NaOH(aq) + Cl2(aq) + H2(aq)
(D) 2NaCl(aq) + 2H2O(l) ¾® 2NaOH(aq) + Cl2(g) + H2(g)
Q.37 Which of the following is not an organic acid?
(A) tartaric acid
(B) oxalic acid [NTSE]
(C) ascorbic acid
(D) nitric acid
Q.38 The sharp pain caused by the sting of an ant is due to [NTSE]
(A) malic acid
(B) nitric acid
(C) formic acid
(D) lactic acid
Q.39 The basicity of an acid is defined as [NTSE]
(A) the number of replaceable OH– ions
(B) the number of H+ ions that can be formed from the acid
(C) the power of the acid to form salts
(D) none of the above
Q.40 Which of the following is not an acidic salt? [NTSE]
(A) CuSO4
(B) Na2CO3
(C) ZnSO4
(D) NH4NO3
Q.41 Soda water has a pH value [NTSE]
(A) > 7
(B) < 7
(C) 7
(D) > 14
Q.42 Many salts absorb water from the atmosphere. This property is called [NTSE]
(A) hydration
(B) dehydration
(C) deliquescence
(D) efforescence
Q.43 Which of the following acid is used by goldsmiths for cleaning gold and silver ornaments?[NTSE]
(A) HCl
(B) H2SO4
(C) HNO3
(D) H3PO4
Q.44 Basic salts are formed by neutralization of [NTSE]
(A) storng acid and strong base
(B) strong acid and weak base
(C) weak acid and weak base
(D) strong base and weak acid
1. D 2. B 3. C 4. A
5. B 6. D 7. B 8. B
9. B 10. A 11. D 12. B
13. D 14. D 15. B 16. D
17. A 18. D 19. A 20. A
21. C 22. C 23. D 24. A
25. A 26. B 27. B 28. A
29. B 30. A 31. C 32. B
33. D 34. B 35. A 36. D
37. D 38. C 39. B 40. B
41. B 42. C 43. C 44. D
1. Which formula represents a salt?
(A) KOH
(B) KCl
(C) CH3OH
(D) CH3COOH
2. Which substance can be classified as an Arrhenius acid?
(A) HCL
(B) NaCl
(C) LiOH
(D) KOH
3. Which solution will change red litmus to blue?
(A) HCl(aq)
(B) NaCl(aq)
(C) CH3OH(aq)
(D) NaOH(aq)
4. An acidic solution could have a pH of
(A) 7
(B) 10
(C) 3
(D) 14
5. What is the pH of a 0.00001 molar HCl solution?
(A) 1
(B) 9
(C) 5
(D) 4
6. What is the pH of a solution with a hydronium
-ion concentration of 0.01 mole per liter?
(A) 1
(B) 2
(C) 10
(D) 14
7. There are alternate acid base theories that define
an acid as any species that can
(A) donate a proton
(B) donate an electron
(C) accept a proton
(D) accept an electron
8. Which 0.1 M solution will turn phenolphthalein pink?
(A) HBr(aq)
(B) CO2(aq)
(C) LiOH(aq)
(D) CH3OH(aq)
9. Given the equation: H+ + OH- <-> H2O. Which
type of reaction does the equation represent?
(A) esterification (B) decomposition
(C) hydrolysis (D) neutralization
10. When HCl(aq) is exactly neutralized by NaOH(aq),
the hydrogen ion concentration in the resulting
mixture is
(A) always less than the concentration of the hydroxide ions
(B) always greater than the concentration of the hydroxide ions
(C) always equal than the concentration of the hydroxide ions
(D) sometimes greater and sometimes less than the concentration of the hydroxide ions
11. As the hydrogen ion concentration of an aqueous solution increases, the hydroxide ion concentration of this solution will
(A) decrease
(B) increase
(C) remain the same
(D) firstly increase then decreases
12. A student wishes to prepare approximately 100
milliliters of an aqueous solution of 6M HCl using
12 M HCl. Which procedure is correct?
(A) adding 50 mL of 12 M HCl to 50 mL of water while stirring the mixture steadily.
(B) adding 50 mL of 12 M HCl to 50 mL of water and then stirring the mixture steadily.
(C) adding 50 mL of water to 50 mL of 12 M HCl while stirring the mixture steadily.
(D) adding 50 mL of water to 50 mL of 12 M HCl and then stirring the mixture steadily.
13. The following data were collected at the endpoint
of a titration performed to find the molarity of
an HCl solution.
Volume of acid (HCl) used = 1(D)4 mL
Volume of base (NaOH) used = 2(B)4 mL
Molarity of standard base (NaOH) = 0.20 M
What is the molarity of the acid solution?
(A) 1.6 M
(B) 0.64 M
(C) 0.31M
(D) 0.13M
14. In general, salts
(A) are ionic compounds
(B) contain hydrogen ions
(C) contain hydroxide ions
(D) turn litmus red
15.When bases ionize they release
(A) hydrogen ions
(B) sodium ions
(C) chloride ions
(D) hydroxide ions
16. A base used in the manufacture of soap is
(A) calcium hydroxide
(B) sodium hydroxide
(C) ammonium hydroxide
(D) zinc hydroxide
17. An element common to all acids is
(A) chlorine
(B) nitrogen
(C) oxygen
(D) hydrogen
18. When magnesium and hydrochloric acid react,
they produce
(A) oxygen and magnesium chloride
(B) chlorine and magnesium oxide
(C) hydrogen and magnesium chloride
(D) hydrogen and magnesium oxide
19. When water solutions of an acid and base are
mixed
(A) no reaction occurs
(B) a new acid and a new base are formed
(C) a salt and water are formed
(D) an acid and a salt are formed
20. A common substance that contains acetic acid
is
(A) vinegar
(B) ammonia water
(C) salad oil
(D) soap
21. Fruit juices, such as orange juice, contain boric
acid
(A) True
(B) False
(C) Both (A) & (B)
(D) None of these
22. When dissolved in water, salts
(A) are nonelectrolytes
(B) have a bitter taste
(C) are electrolytes
(D) release hydrogen ions
23. A base can be prepared by the reaction between
(A) an active nonmetal with water
(B) a gas with water
(C) a sulfide with water
(D) an active metal with water
24. Of the following, the property that most closely
relates to acids is
(A) bitter taste
(B) contains the hydroxide polyatomic ion
(C) sour taste
(D) salty taste
25. when an acid is dissolved in water, it usually forms
(A) hydrogen ions
(B) hydroxide ions
(C) no ions
(D) chloride ions
26. The preparation of hydrochloric acid can be accomplished by heating a mixture of sodium chloride and sodium hydroxide:
(A) True
(B) False
(C) Both (A) & (B)
(D) None of these
27.The acid used in the storage battery in your car is
(A) nitric acid
(B) hydrochloric acid
(C) tartaric acid
(D) sulphuric acid
28. The sour taste of lemons and limes is due to a
substance called
(A) acetic acid
(B) citric acid
(C) hydrochloric acid
(D) carbonic acid
29. A strong acid in solution is
(A) mostly molecules
(B) mostly ions
(C) both molecules and ions
(D) mostly water
30. The pH of a carbonated drink is
(A) less than 7
(B) more than 7
(C) equal to 7
(D) approximately 7.8
31. An acid is
(A) a proton donor
(B) a proton acceptor
(C) an electron donor
(D) an electron acceptot
32. The drying of milk of lime (white washing) is due
to the action of
(A) oxygen in air
(B) nitrogen in air
(C) hydrogen in air
(D) carbondioxide in air
33. A salt derived from a strong base and a weak
acid will give a salt that is
(A) acidic
(B) basic
(C) neutral
(D) volatile
34. When litmus is added to a solution of borax it
turns
(A) red
(B) pink
(C) remain colourless
(D) blue
35. Which of the following is a soluble base in water?
(A) Fe(OH)3
(B) Cu(OH)2
(C) Zn(OH)2
(D) NaOH
36. Which of the following is a weak base
(A) NaOH
(B) KOH
(C) NH4OH
(D) Ca(OH)2