All the materials around us are made up of chemical elements, which are found in the earth crust. Earth is the source of coal, petroleum, graphite, diamond and many other minerals of metals and non-metals. We get various useful things like gasoline, kerosene, wax, coal gas and natural gas from the natural resources, which are made up of many non-metals. These elements occur as minerals and rocks in the earth's crust. Some of these elements like oxygen, nitrogen and carbondioxide occur in atmospheric air.
There are more than 115 elements known at present 80% of these elements are metals and rest are non-metals.
On the basis of their properties , all the elements can be divided into two main groups: metals and non-metals.
Metals are the elements that conduct heat and electricity and are malleable and ductile. Some of the examples of metals are : Iron, Aluminium, Copper, Silver, Gold, Platinum, Zinc.
Metals are the elements which form positive ions by losing electrons (or donating electrons). Metals are known as electropositive elements because they can form positive ions by losing electrons.
The most abundant metal in the earth’s crust is aluminium.
Non-metals are the elements that does notconduct heat and electricity and are neither malleable nor ductile. They are brittle. Some of the examples of non-metals are : Carbon, Sulphur, Phosphorus, Silicon, Hydrogen, Oxygen, Nitrogen. The two allotropic forms of carbon element, diamond and graphite are also non - metal.
Non-metals are the elements which form negative ions by gaining electons. Non-metals are known as electronegative elements because they can form negative ions by gaining electrons.
Carbon is one of the most important non-metals, as life on this earth is based on carbon compound because the carbon compounds like proteins, fats, carbohydrates, vitamins and enzymes etc. are essential for the growth and development of living organisms.
The most abundant non-metal in the earth’s crust is oxygen.
(i) The elements which are placed on the left hand side (except hydrogen) and in the centre of the periodic table are called metals. Such as sodium, potassium, magnesium, calcium, iron, copper zinc etc.
(ii) The elements which are placed on the right hand side of the periodic table are called non-metals such as oxygen, nitrogen, chlorine, fluorine etc. These metals and non-metals are separated from each other in the periodic table by a zig-zag line. The elements placed in the zig-zag line show some properties of metals and some properties of non-metals are called metalloids. Such as boron(B), silicon(Si), germanium(Ge), arsenic(As), antimony(Sb), tellurium(Te) and polonium(Po).
(iii) The position of metals, non-metals and metalloids are shown in a simple form in figure..
Metals present at the extreme left are known as light metals, while those are present in the centre of the periodic table are called heavy metals or transition metals.
(iv) The elements at the extreme left of the periodic table are most metallic and those on the right are least metallic or non-metallic.
Thus, metallic character decreases on going from left to right side in the periodic table. For example, sodium is more metallic than aluminium because sodium is on the left hand side of aluminium.
(v) However on going down in a group the metallic character increases. For example, carbon is non-metal while lead is metal because metallic character increases down in a group.
An element is called metal, which forms positive ions (or cations) by losing electron.
Example : Sodium is a metal which forms sodium ion (Na+) by losing one electron.
Similarly, magnesium metal forms Mg2+ by losing two electrons, Al metal forms Al3+ by losing three electrons.
Thus, metals are also known as electropositive elements.
The atoms of metals have 1 to 3 electrons in their outermost shell. For example, all the alkali metals have one electron in their outermost shell. (Lithium-2, 1, sodium 2, 8, 1, potassium-2, 8, 8, 1, ... etc).
Sodium 11(2, 8, 1) magnesium 12 (2, 8, 2) and aluminium 13 (2, 8, 3) are metals having 1, 2 and 3 electrons respectively in their outermost shell, which lose these electron easily. The number of electrons lost by an atom of a metal is called its valency.
Thus metals have 1 to 3 electrons in their valence shell of their atoms.
Exceptions : Hydrogen and Helium. Hydrogen is a non-metal having 1 electron in its outermost shell of its atom. Helium having 2 electrons in its outermost shell of its atom.
1. Metals are malleable, i.e. metals can be beaten into thin sheets with hammer (without breaking) Malleability is an important property of metals. Gold and Silver metals are some of the best malleable metals. Aluminium foils are used for packing food items like biscuits, chocolates, medicines, cigarettes, etc.
Aim: To test that metals are malleable, i.e. can be hammered into sheets.
Method:
(i) Take piece of iron, zinc, lead and copper.
(ii) Place anyone metal on the block of iron and strike it four or five times with a hammer.
(iii) Repeat with other metals.
(iv) Record the change in shape of these metals.
Discussion and conclusion
It is observed that metals can be beaten into thin sheets, Le., they are melleable.
2. Metal are ductile, that is, metals can be drawn (or stretched) into thin wires.
Ductility is another important property of metals. Gold is the most ductile metal. For example, 1 gram of gold can be drawn into a thin wire about 2 kilometer long. Copper and aluminium metals are also very ductile and can be drawn into thin copper wires and aluminium wires.
Aim: To justify that metals are ductile, i.e., can be drawn into wire.
Method:
(i) Consider some metals such as iron, copper, aluminium, lead etc.
(ii) Check which of these metals are availabte in the form of wire.
Discussion and conclusion
As wires of iron, copper and aluminium are easily available, this shows that metals can be drawn into wires i.e., they are ductile.
3. Metals are good conductors of heat.
Metals allow heat to pass through them easily. Take a flat aluminium rod and stick some nails upon the rod with the help of wax. Start heating the free end of the aluminium rod by keeping a burner below it. We will see that the iron nails attached to aluminium rod with wax start falling one by one because heat travels from the left side to the right side along the aluminium rod. It melts the wax which holds the nails. Silver metal is the best conductor of heat. The cooking utensils and water boilers, etc., are usually made up of copper or aluminium metals because they are very good conductors of heat. Heat conductivity is an important property of metals.
Aim: To test that metals are good conductors of heat and have high melting point.
Method:
(i) Take an aluminium or copper wire. Clamp the wire on a stand.
(ii) Fix a pin to the free end of the wire using wax.
(iii) Heat the wire with a spirit lamp, candle or a burner near the place where it is clamped.
Now answer
(i) What do you observe after some time?
(ii) Does the metal wire melt?
Discussion and conclusion
We observe that on heating the wire near the clamp, after some time the pin falls down. This shows that heat flows
through the wire and melts the wax. Further, the wire does not melt even after heating for a long time.
This shows that metals have high melting points.
4. Metals are good conductors of electricity
Metals allow electricity (or electric current) to pass through them easily. Silver metal is the best conductor of electricity.
The electric wires are made of copper and aluminium metals because they are very good conductors of electricity.
Aim: To test that metals are good conductor of electricity.
Method:
(i) Set up an electric circuit as shown in figure.
(iii) Place the metal to be tested in the circuit between terminals A and B as shown in the figure.
Now Answer
Does the bulb glow? What does this indicate ?
Discussion
The bulb glows. This shows that electric current flows through the metal.
Conclusion
Metals are good conductor of electricity.
5. Metals are lustrous (or shiny) and can be polished
Metals are lustrous, they have a shining surface. For example gold, silver and copper are shiny metals and they can be polished.
The property of a metal having a shining surface is called ‘metallic lustre’.
The metals lose their shine or brightness by keeping in air for a long time and acquire a dull appearance due to the formation
of a thin layer of oxide, carbonate or sulphide on their surface (by the slow action of the various gases present in air).
Aim : To check that metals have lustre, i.e., a shining surface.
Method:
(i) Take samples of iron, copper, aluminium and magnesium. Note the appearance of each sample.
(ii) Clean the surface of each sample by rubbing them with sand paper and note their appearance again.
Discussion: The surface of the metals is dull because they are covered with a layer of oxide,
hydroxide, carbonate etc. due to the attack of gases present in the air on their surface. On rubbing the surface with sand paper
this layer is removed and a shining surface appears.Conclusion: Metals in the pure state (or freshly prepared or cut) have shining surface.
Q. Why do metals possess lustre?
Explanation
When ligth falls on the surface of a metal, the atoms absorb photons as energy. They get excited and start vibrating.
These vibrating electrons release energy in the form of light. Therefore, metal surface shines and metals possess lustre.
6. Metals are generally hard (except sodium and potassium which are soft metals).
Most of the metals like iron, copper, aluminium, etc. are very hard. Some exceptions Sodium and potassium are soft
metals which can be easily cut with a knife.
Activity - 6
Aim: To test that metals are hard and hardness varies from metal to metal.
Method:
(i) Take small piece of iron, copper, aluminium and magnesium. Try to cut these metals with a sharp knife.
(ii) Hold a piece of sodium metal with a pair of tongs.
Caution: Always handle sodium metal with care. Dry it by pressing between the folds of a filter paper.
Put it on a watch glass and try to cut it with a knife.
Discussion and conclusion
All the four metals (Fe, Cu, AI and Mg) are found to be cut with difficulty. This shows that metals are hard. The ease of
cutting is found to be in the order Mg > Al > Cu > Fe. This shows that hardness varies from metal to metal.
Sodium can be cut very easily. Hence sodium is soft, Le., it is an exception.
7. Metals are strong (except sodium and potassium metals which are not strong).
They can hold large weights without snapping (without breaking). For example iron metal (in the form of steel) is very strong.
Due to this iron metal is used in the construction of bridges, buildings,railway lines, machines, vehicles and chains etc.
8. Metals are solid at room temperature (except mercury which is a liquid metal).
9. Metals have high melting points and boiling points (except sodium and potassium metals which have low melting and boiling points)
Example, iron metal has a high melting point of 1535°C. Copper metal has also a high melting point of 1083°C.
Sodium and potassium metals have low melting points (of 98°C and 64°C respectively).
10. Metals have high densities (except sodium and potassium metals which have low densitites)
The density of iron is 7.8 g/cm3 which is quite high. Sodium and potassium metals have low densities (of 0.97 g/cm3 and 0.86 g/cm3 respectively)
11. Metals are sonorous. That is metals make sound when hit with an object.
The property of metals of being sonorous is called sonorousness or sonority. It is due to the property of sonorousness (or sonority)
that metals are used for making bells and strings (wires) of musical instruments like sitar and violin.
12. Metals usually have a silver or grey colour (except copper and gold)
Anodising is a process of forming a thick oxide layer of aluminium. Aluminium develops a thin oxide layer when it exposed to air. This oxide coat of aluminium (Al) make it's resistance to further corrosion. During anodising, the resistance can be improved further by making the oxide layer thicker. In this process, a clean Al article is made the anode and dilute sulphuric acid (H2SO4) is used for electrolyte. The oxygen gas evolved at the anode react with Al to make a thicker protective oxide layer. This oxide layer can be dyed easily to give Al articles an attractive finishing.
(i) Many metals and their compounds are useful in our daily life. These are as follows : Aluminium is used to prepare utensils and house hold equipments like vacuum cleaner. Aluminium is extensively used in making bodies of rail, cars, automobiles, trucks and aircraft. Aluminium wires are widely used in electrical work. Aluminium foil is used to wrap chocolate cigarette and medicines and to seal milk bottles.
(ii) Major use of copper is in making electrical wires & cables. Copper is also used in making utensils, steam pipes, coin and in electroplating.
(iii) Steel is an alloy of iron which is used for making parts of machines, as building material and in the construction of refrigerator. As a matter of fact steel is said to be the back bone of industry.
(iv) Gold and silver called noble metals (or coinage metals) are used in jewellery.
(v) Mercury is used in thermometers barometers and to prepare amalgams.
(vi) Platinum is used to make crucibles and electrodes.
(vii) Zinc is used to galvanize iron, to prepare roofing material, container of dry cells and to make brass when mixed with copper.
(viii) Metal like sodium, titanium and zirconium find their applications in atomic energy, research and medical industry.
(ix) Titanium (Ti) and its alloys are used in aerospace, marine equipments, aircraft frames, chemical industries and chemical reactors.
The wide application of titanium is attributed to its resistance to corrosion, high melting points and high strength.
1. Reaction of Metals with oxygen (Air)
When metals are burnt in air, they react with the oxygen of air to form metal oxides :
![]()
From air (Basic oxide)
Metals react with oxygen to form metal oxides. Metal oxides are basic in nature. The vigour of reaction with oxygen
depends on the chemical reactivity of metal.
(i) Sodium metal reacts with the oxygen at room temperature to form a basic oxide called sodium oxide:
4Na(s) + O2(g) ¾¾® 2Na2O(s)
sodium oxygen sodium oxide
(Metal) (from air) (Basic oxide)
Potassium metal and sodium metal are stored under kerosene oil to prevent their reaction wilh the oxygen,
moisture and carbon dioxide. Some of the metal oxides dissolve in water to form alkalies.
Eg![]()
(ii) Magnesium metal does notreact with oxygen at room temperature. But on heating, magnesium metal burns in
air giving instense heat and light to form a basic oxide called magnesium oxide (which is a white powder)
![]()
Magnesium Oxygen Magnesium oxide
(Metal) (From air) (Basic oxide)
Magnesium oxide dissolves in water partially to form magnesium hydroxide solution :
![]()
(iii) Aluminium metal burns in air on heating to form aluminium oxide :
![]()
Those metal oxides which show basic as well as acidic behaviour are known as amphoteric oxides. Aluminium metal and zinc metal
form amphoteric oxides. Amphoteric oxides react with both, acids as well as bases to form salts and water. Example :
![]()
![]()
(iv) Zinc metal burns in air only on strong heating to form zinc oxide :
![]()
Zinc oxide reacts with hydrochloric acid to form zinc chloride (salt) and water.
![]()
(v*) Iron metal does notburn in air even on strong heating. Iron reacts with the oxygen on heating to form iron (II, III) oxide :
![]()
(vi*) Copper metal also does notburn in air even on strong heating. Copper reacts with the oxygen on prolonged heating to form a black substance copper (II) oxide :
![]()
Generally, metallic oxides are basic in nature except aluminium and zinc oxides which are amphoteric in nature. This means these oxides (Al2O3, ZnO) react with base as well as acid. The basic oxide of metals react with acid to give salt.
For example :
![]()
Some oxide of metals dissolve in water and form alkalis.
Example for :
![]()
Sodium hydroxide
![]()
Potassium hydroxide
Reaction showing amphoteric in nature of Al2O3 and ZnO.
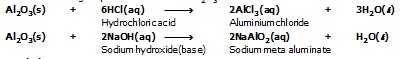
Sodium hydroxide(base) Sodium meta aluminate
Similarly,

2. Reaction of Metals with water
Metals react with water to form a metal hydroxide (or metal oxide) and hydrogen gas.
(i) Potassium react violently with cold water to form potassium hydroxide and hydrogen gas :
![]()
(ii) Sodium reacts vigorously with cold water forming sodium hydroxide and hydrogen gas:
![]()
(iii) Calcium reacts with cold water to form calcium hydroxide and hydrogen gas :'
![]()
The piece of calcium metal starts floating in water because the bubbles of hydrogen gas formed during the reaction stick to its surface.
(iv) Magnesium metal does not react with cold water. Magnesium reacts with hot water to form magnesium hydroxide and hydrogen :
![]()
In this reaction the piece of magnesium metal starts floating in water due to the bubbles of hydrogen gas sticking to its surface.
Magnesium reacts very rapidly with steam to form magnesium oxide and hydrogen :
Mg (s) + H2O(g) ¾¾® MgO(s) + H2(g)
Magnesium Steam Magnesium oxide Hydrogen
(v) Aluminium reacts with steam to form aluminium oxide and hydrogen gas :
![]()
Aluminium metal does notreact with water under ordinary conditions because of the presence of a thin (but tough) layer of aluminium oxide on its surface.
(vi) Zinc reacts with steam to form zinc oxide and hydrogen :
![]()
(vii) Red - hot iron reacts with steam to form iron (II, III) oxide and hydrogen :
![]()
Metal like lead, copper, silver and gold does not react with water (or even steam).
Aim: To study the reactivity of metals with water.
Caution: This activity needs teacher’s assistance.
Method:
(i) Collect the samples of sodium, potassium, calcium, magnesium, zinc and copper.
(ii) Put small piece of the samples separately in beakers half-filled with cold water.
(iii) Put the metals that do not react with cold water in beaker half-filled with hot water.
(iv) For the metals that do not react with hot water, arrange the apparatus (to produce steam) and observe their reaction with steam.
Now Answer
(i) Which metals reacted with cold water? Arrange them in increasing order of their reactivity with cold water.
(ii) Does any metal produce fire on water ?
(iii) Does any metal start floating after some time ?
(iv) Which metals did not react even with steam ?
Discussion
(i) Na and K metals react vigorously with cold water to form NaOH and H2 gas is liberated.
![]()
(ii) 2K(s) + 2H2O(l) ¾® 2KOH(aq) + H2(g)
Potassium Cold water Potassium hydroxide Hydrogen gas
The reactions are so violent and exothermic that the H2 gas evolved catches fire.
(iii) Calcium reacts with cold water to form Ca(OH)2 and H2 gas. It is less violent.
![]()
(iv) Magnesium react with hot boiling water to form MgO and H2 gas.
![]()
(v) Aluminium does not react either with cold or hot water. But it react only with steam to form aluminium oxide and hydrogen gas.
![]()
(vi) Similarly, zinc react with steam to form zinc oxide and H2 gas.
![]()
(vii) Copper do not react with water even under strong conditions. The above reactions indicate that sodium and potassium are the most reactive metals while copper is less reactive.
Conclusion
The reactivity order of these metals with water are
K > Na > Ca > Mg > Al > Zn > Fe > Cu
3. Reaction of metals with Dilute Acids :
Metals usually displace hydrogen from dilute acids. When a metal reacts with a dilute acid, then a metal salt and hydrogen gas are formed
Metal + Dilute acid ¾¾® Metal salt + Hydrogen
(i) Sodium metal reacts violently with dilute hydrochloric acid to form sodium chloride and hydrogen:
![]()
(ii) Magnesium reacts quite rapidly with dilute hydrochloric acid forming magnesium chloride and hydrogen gas :
![]()
(iii) Aluminium metal at first reacts slowly with dilute hydrochloric acid due to the presence of a tough protective layer of aluminium oxide on its surface.But when the thin, outer oxide layer gets dissolved in acid.
Aluminium metal reacts rapidly with dilute hydrochloric acid to form aluminium chloride and hydrogen gas :
![]()
The reaction of aluminium with dilute hydrochloric acid is less rapid than that of magnesium, so aluminium is less reactive than magnesium.
(iv) Zinc reacts with dilute hydrochloric acid to give zinc chloride and hydrogen gas(but the reaction is less rapid than that of aluminium)
![]()
This reaction shows that zinc is less reactive than aluminium.
(v) Iron reacts slowly with cold dilute hydrochloric acid to give iron (II) chloride and hydrogen gas:
![]()
(vi) Copper does notreact with dilute hydrochloric acid (or dilute sulphric acid) at all. This shows that copper is even less reactive than iron :
![]()
Metals like copper and silver which are less reactive than hydrogen, does notdisplace hydrogen from dilute acids.
When a more reactive metal is placed in a salt solution of less reactive metal, then the more reactive metal displaces the less reactive metal from its salt solution . This reaction is also known as displacement reaction. Let us learn it with the help of following activity.
Aim : To compare the reactivity of the metals.
Procedure : Take a clean wire of copper and an iron nail and two test tube. Now dissolve copper sulphate in water in one test tube and ferrous sulphate in another test tube. Place iron nail in the blue coloured copper sulphate solution with the help of a thread and copper wire in the greenish colour ferrous sulphate solution as shown in figure as below.
Observation : The blue colour of copper sulphate has faded and becomes greenish. The green colour of the solution is due to the formation of iron (II) sulphate and copper is displaced. A reddish-brown coating is formed on the surface of iron nail. The reaction is represented by the chemical equation.
![]()
But the greenish colour of FeSO4 does notchange. That means no reaction take place.
Conclusion : These activities shows that iron metal is more reactive than copper.
Similarly,
When a strip of copper metal is placed in a solution of AgNO3. The solution becomes gradually blue and a shining coating of silver metal gets deposited on the copper strip. The reaction may be written as :
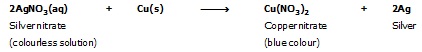 However, if we place silver wire in a copper sulphate solution no reaction occurs. This means copper can displace silver from its salt solution but silver cannot displace copper from its solution. i.e. copper is more reactive metal than silver.
However, if we place silver wire in a copper sulphate solution no reaction occurs. This means copper can displace silver from its salt solution but silver cannot displace copper from its solution. i.e. copper is more reactive metal than silver.

Non-metals are present on the right hand side of the periodic table (exception : Hydrogen). Among the total known elements, there are only 22 non-metals, out of which 11 are gases like oxygen, nitrogen, hydrogen one is a liquid (Bromine) and the rest 10 are solids such as sulphur, phosphorus and the allotrops of carbon (Diamond and graphite).
An elements is called non-metal which form ions by gaining electrons. For example, oxygen is a non-metal which form O2– ions by gaining two electrons. Similarly, nitrogen form N3– ions by gaining three electrons.
Thus, non-metals also known as electronegative elements.
The atoms of non-metals have usually 4 to 8 electrons in their outer most shell. For example,
Carbon (At No. 6), Nitrogen (At. No. 7), Oxygen (At. No. 8), Fluorine (At. No. 9) and Neon (At. No. 10), have respectively 4, 5, 6, 7 and 8 electrons in their outermost shell. However, there are two exceptions namely hydrogen and helium which have one and two electrons in their valence shell or outer most shell, but they are non-metals.
1. Non-metals are neither malleable nor ductile. Non-metals are brittle (break easily).
Solid non-metals can neither be hammered into thin sheets nor drawn into thin wires. For example, sulphur and phosphorus are solid non-metals which are non-malleable and non-ductile. The property of being brittle (breaking easily) is called brittleness. Brittleness is an important property of non-metals.
2. Non-metals does not conduct heat and electricity.
Non-metals does notconduct heat and electricity because unlike metals, they have no free electrons (which are necessary to conduct heat and electricity). For example, sulphur and phosphorus are non-metals which does notconduct heat and electricity. There is, however one exception, carbon (in the form of graphite) is the only non-metal which is a good conductor of electricity because of it’s structure.
3. Non-metals are not lustrous (not shiny). They are dull.
Non-metals does nothave a shining surface. For example, sulphur and phosphorus are non-metals which have non lustre. Iodine is a non-metal having lustrous appearance.
4. Non-metals are generally soft (except diamond which is extremely hard non-metal)
5. Non-metals are not strong. They are easily broken.
6. Non-metals may be solid, liquid or gases at the room temperature.
7. Non-metals have comparatively low melting points and boiling points (except diamond which is a non-metal having a high melting point and boiling point).
The melting point of sulphur is 115°C which is quite low. The melting point of diamond is, however more than 3500°C which is very high.
8. Non-metals have low densities, that is, non-metals are light substances.
The density of sulphur of 2g/cm3.
9. Non-metals are non-sonorous. They does not produce sound when hit with an object.
10. Non-metals have many different colours.
On the basis of the above discussion of the physical properties of metals and non-metals, we have concluded that elements can not be grouped according to the physical properties alone, as there are many exceptions.
For example,
(i) All metals except mercury are solids at room temperature. We know that metals have very high melting points but gallium (Ga) and caesium (Cs) have very low melting points. These two metals will melt if we keep them at our palm.
(ii) Iodine is a non-metal but it is lustrous.
(iii) Alkali metals such as Lithium, Sodium and Potassium are soft and they can be easily cut with a knife. i.e. they have very low densities and low melting points.
(iv) Carbon is a non-metal that can exist in different forms. Each form is called an allotrope of Diamond, an allotrope of carbon is the hardest natural substance. which has very high melting and boiling point. Graphite is another allotrope of carbon which is good conductor of electricity.
The elements can be more clearly classified as metals and non-metals on the basis of their chemical properties.
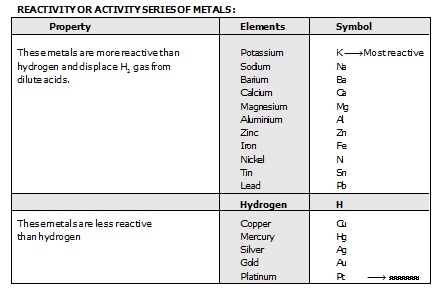
The arrangement of metals in order of decreasing reactivities is called reactivity series or activity series of metals. After performing displacement experiments the following series has been developed.
(i) The most reactive metal is placed at the top and the least reactive metal is placed at the bottom of the table.
(ii) Metals present above the hydrogen in reactivity series can displace hydrogen from dilute acids.
(iii) A metal can displace the metals placed below it in the reactivity series.
(iv) Metals present at the top are more elecro-positive, so they will occur in combined or compound form only in nature.
(v) Metals at the bottom are less reactive and do not react easily so they may be present in free state in nature.
Ex.1 A, B and C are three elements which undergo chmical change according to the following equations :
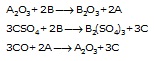
Write the anme of the most reactive and the least reactive elements.
Sol. (i) In the first reaction, B displaces A, so B is more reactive than A.
(ii) In second reaction, B displaces C, so B is more reactive than C.
(iii) In third reaction, A displaces C, so A is more reactive than C.
So, B is more reactive than A and C and A is more reactive than C, So the order of their reactivities is as follows:
B > A > C
Ex.2 Explain why zinc metal can displace copper from copper sulphate solution but copper cannot displace zinc from zinc sulphate solution.
Or
When a piece of copper metal is added to a solution of zinc sulphate, no change takes place, but the blue colour of copper sulphate fades away when a piece of zinc is placed in its solution.
Sol. When a piece of zinc is placed in a solution of copper sulphate, zinc being more reactive than copper, can displace copper from its salt solution and forms zinc sulphate and blue colour of copper sulphate fades away slowly, but when a piece of copper sulphate fades away slowly, but when a piece of copper metal is added to a solution of zinc sulphate, no change takes place as copper being less reactive than zinc, cannot displace zinc from zinc sulphate.
electronic configuration of some elements :
Types of element Element Atomic number Number of electrons in shells
K L M N
Noble gases Helium (He) 2 2
Neon (Ne) 10 2 8
Argon (Ar) 18 2 8 8
Metals Sodium (Na) 11 2 8 1
Magnesium (Mg) 12 2 8 3 Aluminium (Al) 13 2 8 3
Potassium (K) 19 2 8 8 1
Calcium (Ca) 20 2 8 8 2
Non-Metals Nitrogen (N) 7 2 5
Oxygen (O) 8 2 6
Fluorine (F) 9 2 7
Phosphorus (P) 15 2 8 5
Sulphur (S) 16 2 8 6
Chlorine (Cl) 17 2 8 7
It is clear from the above table that except helium, all other noble gases have 8 electrons (octet) in their outermost shell. Which represent a highly stable electronic configuration. Due to this stable configuration, the noble gases have no any tendency to lose or gain electrons. So they exist monoatomic, sodium atom has one electron in its outermost shell. If it loses the electon from its M shell the its L shell becomes the outermost shell. which has stable octet like noble gases. The nucleus of this atom still has 11 protons but the number of electrons has 10. Therefore, if becomes positively charged sodium ion or cation (Na+).

On the other hand chlorine has seven electrons in its outer most shell and it require one more electron to complete its octet. The nucleus of chlorine atom has 17 protons and the number of electrons become 18. This makes chloride ion, Cl– as negatively charged
![]()
So, Na+ and Cl– ions being oppositely charged atoms which attract each other and are held by strong electrostatic forces of attraction to exist as NaCl. In other words, Na+ and Cl– ions are held together by electrovalent or ionic bond.
The formation of one more ionic compound magnesium chloride :
The electronic configuration of magnesium (Mg) and chlorine atoms are :
![]()
Magnesium atom has two electrons in its valence shell. It has a tendency to lose both of its electrons to attain the nearest noble gas configuration (i.e. Ne). Mg ¾® Mg2+.
On the other hand, chlorine has only one electron less than the nearest noble gas (i.e. Ar) configuration. The magnesium loses its both the valence electrons to two chlorine atoms, each of which is need of one electron to form Cl– ion.
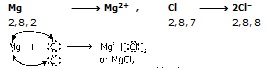
The compounds formed by the transfer of electrons from a metal to a non-metal are known as ionic compound or electrovalent compounds. The structure of some common ionic compounds are given below :

Aim: To study the properties of ionic compounds.
Method:
(i) Take samples of sodium chloride, potassium iodide, barium chloride or any other salt from the science laboratory.
(ii) Take a small amount of sample on a metal spatula and heat directly on the flame. Repeat with other samples.
(iii) Try to dissolve the compound in water and kerosene.
(iv) Make a circuit as shown in figure and insert the electrooes into a solution of one salt. Test the other salt samples too in this manner.
Now Answer
(i) What is the physical state of the salt taken ?
(ii) Did the samples impart any colour to the flame on heating ?
(iii) Did the compounds melt on heating?
(iv) Are the compounds soluble in water or kerosene ?
(v) Did the electric bulb glows on passing electric current ?
(vi) What is your inference about the nature of these compounds?
Discussion
(i) All the salts taken are solids. Each salt imparted a particular colour to the flame.
(ii) The compounds did not melt on heating.
(iii) The compounds were soluble in water but not in kerosene.
(iv) The electric bulb glows on passing electric current. All these properties show that the compounds are ionic in nature.
Conclusion
(i) Ionic compounds are generally solids.
(ii) They impart a characteristic colour to the flame.
(iii) They are soiuble in a polar solvent like water and insoluble in non-polar solvent like kerosene, petrol, etc.
(iv) Their molten or aqueous solution conduct electricity.
(a) Physical state
Ionic compounds are solids and relatively hard because of the strong force of attraction between the positive and negative ions. This force of attraction is also known as strong electrostatic force of attraction. These compounds are generally brittle and break into pieces when pressure is applied.
(b) Solubility
Electrovalent compounds are generally soluble in water (because of their polar nature) and insoluble in solvents such as kerosene, petrol, etc.
(c) Melting and boiling points
Ionic compounds have high melting and boiling points, due to the strong electrostatic force of attraction belween the oppositely charged ions. Therefore, large amount of energy is needed to break these bonds.
(d) Conduction of electricity
Ionic compounds in the solid state do not conduct electricity because movement of ions in the solid state is not possible due to their rigid structure. But they can conduct electricity in molten or aqueous state.
(e) Colour to the flame
Most of the salts when brought into the flame, impart characteristic colour to the flame.
Hydrogen gas is not evolved when metals such as Zn, Fe, Cu and Al reacts with nitric acid. Because HNO3 is strong oxidising agent. It oxidises H2 gas to water and itself gets reduced to form oxides of (NO, N2O and NO2) nitrogen.

But copper reacts with hot concentrated sulphuric acid (H2SO4) to produce copper sulphate, sulphur dioxide and water.
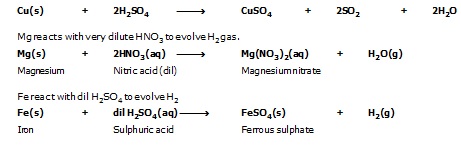
Aqua regia is a Latin word it means " royal water". It is a freshly prepared mixture of concentrated hydrochloric acid and concentrated nitric acid in the ratio of 3 : 1. It is a highly corrosive, fuming liquid and it is used to dissolve gold and platinium.
The main source of metal is earth’s crust. Some metals also occur in sea water. The metals are found in nature in :
(1) Native state (or free state) : Only a few less reactive metals like silver, gold platinum etc., are found in the free state in which they are called “native metals”.
(2) Combine state : i.e., in the from of their compounds admixed invariably with various useless impurities such as clay, sand, rocky material, etc. Usually, metals are found in the form of oxides, sulphides, carbonates, phosphates, halides silicates, etc.
(i) The naturally occurring form of metal in combined state, is known as “mineral”.
(ii) Those naturally occurring minerals, which are economically suitable for commercial extraction of metals, are known as ‘ores’. Thus, every ore is a mineral, but every mineral is not an ore.
(iii) The rocky and earthy impurities (like clay, sand) generally associated with ore, are called gangue (or matrix).
TYPES OF ORES
Note :
(1) Sodium a very reactive metal, and reacts readily with moisture, oxygen and carbon dioxide of air. So sodium cannot exit ‘free’ in nature. Hence, it is not found ‘native’ in nature.
(2) Sodium is highly reactive metal, and has affinity for oxygen. If it is exposed to air, a coating of the oxide is formed and sometimes, it may even catch fire. Consequently, sodium metal should not be exposed to air. Hence, sodium is stored under kerosene.
(3) Aluminium is a reactive metal, so it is not found in free state in nature. It occurs in the form of its compounds, chief of which is bauxite (Al2O3 2H2O).
(4) Gold and silver occupr low position in the activity series. Consequently, they are least reactive elements and are not effected by most chemicals, atmospheric oxygen, moisture, carbon dioxide etc. Hence, they often occur in free or native state in nature.
Extraction of metals : We have learnt about the reactivity series of metals, according to which, the metals at the “bottom” of the reactivity series are the “least reactive” and these are often found in a free-state, e.g., Au, Ag, Pb and Cu. However Cu and Ag are also found in combined state as their oxides and sulphides. On the other hand, metals at the “top” of the reactivity series are so reactive, they are never found in nature as free elements, e.g., Li, K, Na, Ca, Mg etc. The metals in the “middle” of the reactivity series (e.g., Al, Zn, Fe, Pb etc.) are moderately reactive and they are found in the earth’s crust mainly as oxides, sulphides or carbonates [e.g., Al2O3. 2H2O (bauxite), HgS (cinnaber), ZnCO3 (calamine)].
On the basis of reactivity seires, we can have following three groups of elements :
(i) Metals of low reactivity.
(ii) Metals of medium rectivity.
(iii) Metals of high reactivity.
Metallurgy : is the process of extracting a metal in the free form from its ore and then refining it for use. Various steps involved in the extraction of metals from their ores are generally as follows:
(a) Concentration (or enrichment) of ore
(b) Conversion of concentrated ore into oxide
(c) Reduction of oxide ore into impure metal
(d) Refining of impure metal.
(a) Con centration (or enrichment) of ore : The ore is, generally, associated with useless rocky and earthy impurities (like clay, sand etc.), called ‘gangue’ or matrix. The ‘concentration’ (or enrichments) of ore means removal of gangue from the powdered ore. Thus, the percentage of the metal in the concentrated ore is higher than that in the original ore. The concentration of ore can be brought about in the following ways. depending upon the type of ore such as hydraulic washing, froth floatation method, magnetic separation etc.
(i) Levigation or gravity separation or hydraulic washing
This method is based upon the difference in the densities of the ore particles and impurities (gangue). Example: Haemetite ore of iron.
(ii) Froth floatation
This method is based on the difference in the wetting properties of the ore and gangue particles with water and oil. It is used for enrichment of sulphide ores. Example: ZnS, HgS.
(iii) Liquation
This method is based on difference in melting point of ore and gangue particles. Example: ore of tin and zinc.
(iv) Magnetic separation
This method is based on difference in the magnetic properties of the ore and gangue. Example: magnetite (Fe3O4) ore of iron.
(v) Chemical separation
When none of the physical propertry makes the difference, then we use chemical properties as the basis for enrichment. e.g. Bayer’s process for alumina enrichment.
Next steps of metallurgy depend on the type of metal to be extracted :
(a) Extracting metals low in reactivity series : Since these metals are unreactive, so the oxides of these metals can be “reduced” by heating alone. For example, cinnabar (HgS) an ore of mercury changes to mercury on heating
(b) Extracting metals in the middle of the reactivity series : Since these metals (e.g. Fe, Zn, Pb, Cu, etc.) are moderately reactive, so they are usually found in earth’s crust as sulphides or carbonates.
Consequently are converted into metal oxides.
(i) The process of conversion of metal sulphide to oxide by strongly heating in the presence of excess air, is called roasting. For example :
![]()
(ii) The proces of conversion of metal carbonate to oxide by heating strongly in limited air, is called calcination. For example :
Reduction of oxide to metal : The metal oxides obtained above are reduced by hearting with suitable reducing agents like carbon. For example :
![]()
It may be pointed out here that besides using carbon (coke), to reduce metal oxides to metals, sometimes, displacement reactions are also employed. The highly reactive metals (e.g., Na, Ca, Al, etc.) are employed as reducing agents, since they displace metals of lower reactivity from their compounds, For example :
![]()
Such a displacement reaction is highly exothermic (i.e., lot of heat is evolved), so the metal produced is in molten state [e.g., Mn(l)] Al is also used to reduce iron (III) oxide (Fe2O3) and this reaction is called thermite reaction and used to join railway trackes or machine parts.
![]()
(c) Extracting metals near the top of the reactivity series : Since these metals are highly reactive, so their oxides cannot be reduced by heating with carbon. For example, Na2O(s), MgO(s), CaO(s), Al2O3(s), etc. cannot be reduced by heating with carbon. This is because these metals possess more affinity for oxygen than carbon. Consequently, these metals are extracted by electrolytic reduction process. For example, when molten sodium chloride is electrolyed sodium is obtained at the cathode (the negatively charged electrode) : while chlorine is liberated at the anode (the positively charged electrode).
Thus : At cathode : Na+ + e– —® Na(s)
At anode : 2Cl– —® Cl2 + 2e–
Likewise, Al is obtained by the electrolytic reduction of Al2O3.
(d) Refining of metals : the process of purifying the crude metal to get pure metal, is called refining. The method of metal refining depends on :
(i) the nature of the metal to be purified and (ii) the type of impurities present.
Electrolytic refining : Most of the metals are refined by this method. In this process, a large block of impure metal is made the anode in an electrolytic cell, and a thin sheet of pure metal is made the cathode. Suitable metal salt solution is made as an electrolyte. On passing electric current, pure metal deposits on the cathode sheet; while some of impurities are left in solution, and other noble metal impurities settle below the anode as ‘anode mud’.
For exmple, during the electrolytic refining of a copper, a thick block of impure copper is made anode, and thin plate of pure copper is made cathode. Copper sulphate solution. is used as an electrolyte.
On passing electric current, following reactions take place :
(1) Cu2+ ions (from copper sulphate solution) go to the cathode (negative electrode), where they are reduced to copper, which gets deposited on the cathode.
(2) Copper (of impure anode) forms copper ions and these go into solution of electrolyte.
Thus, the net result is transfer of pure copper from anode to the cathode. Impurities like zinc, iron etc., go into solution; while noble impurities like silver, gold etc., are left behind as anode mud.
Any process of deterioration (or destruction) and consequent loss of a solid metallic material, through an unwanted (or unintentional) attack by its environment, starting at its surface, is called corrosion. Thus, corrosion is a proces “reverse of extraction of metals”.
The most familiar example of corrosion is rusting of iron, when exposed to the atmospheric conditions. During this, a layer of reddish scale and powder of oxide (Fe2O3 . x H3O) is formed and the iron becomes weak. Another common example is formation of green films of basic copper, when exposed to moist-air containing carbon dioxide. Similarly, silver article turns black after some time, when exposed to air. This is due to the reaction of Ag with H2S present in air to form black coloured Ag2S.
Note :
(i) It may be pointed out that noble metals such as gold and platinum do not corrode easily.
(ii) The process of corrosion is continuous and causes decrease in strength of the metal.
Prevention of rusting :
(i) By painting: The corrosion of a metal can be prevented simply by painting the metal surface by grease or varnish taht forms a protective layer on the surface of the metal which protect the metal from moisture and air.
(ii) Self prevention: Some metals form protective layers.
For example: When zinc is left exposed to the atmosphere, it combines with the oxygen of air to form a layer of zinc oxide over its surface. The oxides layer does not allow iar to go inside the metal. Thus, zinc is protected from corrosion by its own protective layer.
Similarly, aluminium combines with oxygen to form a dull layer of aluminium oxide on its surface which protect the aluminium from further corrosion.
(iii) Cathodic protection: In this method the more reactive metal which is more corrosion-prone is connected to a bar of another metal which is less reactive and to be protected. In this process electron flow from the more reactive metal to the less reactive metal. The metal to be protected becomes the cathode and the mroe reactive metal becomes the anode.
In this way, the two metals form an electrochemical cell and oxidation of the metal is prevented.
Example: The pipelines (iron) under the surface of the earth are protected from corrosion by connecting them to a more reactive metal (magnesium or Zn) which buried in the earth and connected to the pipelines by a wire.
(vi) Oiling and greasing: Both protect the surface of metal against moisture and chemicals etc. In addition the oil and grease prevent the surface from getting scratched.
(v) Electroplating: It is a very common and effective method to check corrosion. The surface of metal is coated with chromium, nickel or aluminium etc. by electrolysis also called electroplating. They are quite resistant to the attack by both air and water and check corrosion. If the surface of metal is electroplated by zinc, it is known as galvanisation and in case tin metal is used, then the process is called tinning.
(vi) By alloying: It is a very good method of improving the properties of a metal.
For example: Iron is the most widely used metal. But it is never used in its pure state. This is because pure iron is very soft and stretcheds easily when hot. But, it it is mixed with a small amount of carbon (about 0.05%) it becomes hard and strong.
When iron is mixed with nickel and chromium to form stainless steel which is hard and does not rust, i.e. its properties change. In fact, the properties of any metal can be changed, if it is mixed with some other subtances.
Importance of corrosion : Sometimes corrosion of a metal prevents further corrosion of the underlying metal : For example, when Al is exposed to air a thin coating of Al2O3 on the metal article is formed. This film, quite adhering and non-porous, thereby it protect the Al metal underneath from further corrosion and damage. This is the reason why Al, being a very reactive metal, is used for making uternsils.
“An alloy is a homogeneous solid solution of one metal with one or more metals or non-metals.” such as brass, bronze, steel etc.
Purposes of alloy making : Alloys are generally, made to serve one or more of the following purposes:
(i) To modify chemical activity such as increased resistance to corrosion.
(ii) To harden a metal e.g., copper in gold ornaments.
(iii) To increase the strength and toughness.
(iv) To lower the melting point.
(v) To produce good castings.
For instance, pure iron is very soft and stretches easily, but it is mixed with some metals and non-metals, the alloys formed show considerable improvement in the qualities.
(i) Steel : When iron has carbon (0.05 to 0.5%) it is called steel. It is hard and strong. It is used for making ships, vehicles and building.
(ii) Stainless Steel : When steel is mixed with nicked and chromium, it is called stainless steel. It is hard and rust-proof. It is used for making utensils, equipments for feed and dairy industry.
Some common Alloys
(i) Brass : It is an alloy of copper and zinc (Cu-60 to 90%; Zn-10 to 40%). It is a yellow coloured alloy and used for making utensils, coins and decorative pieces.
(ii) Bronze : It is an alloy of copper and tin (Cu-88 to 96%; tin-4 to 12%). It is shining light, yellowish coloured alloy. It is used for making statures, ships and medals.
(iii) Solder : It is an alloy of lead and tin (lead 33%; tin 67%). Its melting point is low. It is used for soldering electrical wires.
(iv) Alloying of gold : The purity of gold is expressed in ‘carat’ and 24 carat gold is supposed to be 100% pure. Pure gold or 24 carat gold tis very soft and cannot be sued for making ornaments. To make is hard, it is alloyed with silver, copper or both. Mosdy 22 carat or 20 carat gold is used for making ornaments. 22 carat gold means 22 parts of pure gold mixed with 2 parts of silver or copper or both.
(v) Duralumin: It is an alloy of aluminium. It contains 95% of aluminium, 4% of copper, magnesium is 0.5% and 0.5% of manganese. It is stronger and lighter than aluminium. Duralumin is used for making bodies of air crafts, helicopters, jets, kitchen ware like pressure cooker. It is also used for making bodies of ships (due to its resistance to sea water corrosion). It is also known as duralium.
(vi) Amalgam: It is an alloy of mercury and one or more other metals is known as an amalagam. It may be solid or liquid. A solution of sodium metal in liquid mercury metal is called sodium amalgam, which is used as a reducing agent. Amalgam of silver, tin and zinc is used by dentists for filling in teeth.
Ex.1 What determines the reactivity of metals ?
Sol. If a metal atom lose one or more electrons easily to form positive ions, it will react readily with other substance. Thus, a more electropositive element is more reactive and a less electropositive element is less reactive. Electropositive character of a metal determines its reactivity.
Ex.2 Write a short note on a reactivity series of metals ?
Sol. Some metals are very reactive while others are less reactive or does not react at all. For example, sodium and potassium reacts very vigurously even with cold water, so they can be said to be very reactive metals. Zinc and iron does not reacts with hot water, but reacts with steam, so they are less reactive metals. On the other hand, copper and silver does not reacts even with steam, so they are quite unreactive metals. Thus, on the basis of vigurousity with which various metals react with oxygen, water and acids the metals have been arranged in a series. The below arrangement of metals in a vertical column in decreasing order of reactivity is called the activity series of metals.
Reactivity or Activity Series of Metals :
Ex.3 Explain, why some metals are more reactive and others less reactive ?
Sol. When metals react, they lose electrons to form positive ions. Such metals which can lose electrons easily to form positive ions when reacted with other substance, are reactive metals. On the other hand, if a metals loses electrons less rapidly as compare to more reactive metal to form positive ions, it will react slowly with other substances. Such a metal will be less reactive.
Example : Sodium atom is a very reactive metal because it readily loses one electrons, forms a positive ion which then combines with other substances. On the other hand, lead atom loses electrons with difficulties to form positive ions, so lead metal is less reactive.
Ex.4 What happen when :
(i) Lead is heated to 400ºC–500°C in air. (ii) Steam is passed over heated iron.
(iii) Copper oxide is heated with magnesium. (iv) Aluminium wire is dipped in heating water.
Sol. (i) Lead forms litharge and red lead when heated in air.
![]()
(ii) Red hot iron displaces hydrogen from steam.
![]()
(iii) On heating with magnesium, copperoxide is reduced to the copper metal.
![]()
(iv) In boiling water, aluminium forms aluminium hydroxide and H2 gas is liberated.
![]()
Ex.5 How would you show that silver is chemically less reactive than copper ?
Sol. Take copper sulphate solution in a test-tube and place a silver plate in it. After some time there is no change in the test tube, i.e., blue colour of copper sulphate does not fade away. This shows silver is less reactive than copper.
Ag(s) + CuSO4 (aq) ¾® No reaction
Ex.6 State the important physical properties of non metals.
Sol. The important physical properties of non metals are given below :
(i) Non metals are brittle, i.e., they cannot be beaten into sheets. When hammered they break into pieces. For example, sulphur and phosphorus are brittle non - metals.
(ii) Non metals are non ductile, i.e., they cannot be drawn into thin wire on stretching.
(iii) Non-metals are bad conductors of heat and electricity. Except carbon (in the form of graphite) non -metals does not conduct heat and electricity because unlike metals they have no free electrons.
(iv) Non - metals are dull. Except iodine and graphite, non-metals have no lustre (shine).
(v) Non - metals have comparatively low m.p. and b.p.
(vi) Non - metals have low densities.
(vii) Non- metals may be solid, liquid or gas at room temperature.
Carbon, sulphur and phosphorus are solid non-metals, bromine is a liquid non - metals ; hydrogen, oxygen and nitrogen are gaseous non-metals.
(viii) Most solid non metals are soft. Only carbon (in the form of diamond) is very hard.
(ix) Non-metals are not strong ; i.e., these have low tensile strength.
Ex.7 List six differences in the physical properties of metals and non-metals ?Sol.
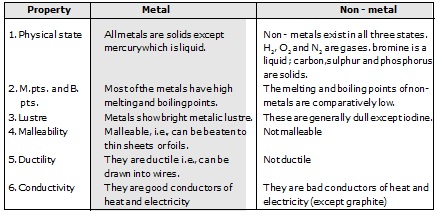
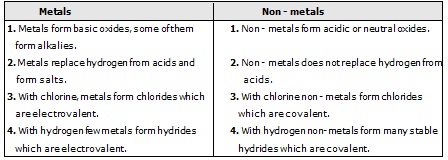
Ex.8 Give a comparison of chemical properties of metals with those of non - metals.
Sol. Chemical Properties :
Ex.9 Write a note on the “solubility” of ionic compounds ?
Sol. Ionic compounds are generally soluble in polar solvents like water because molecules of the polar solvents strongly interact with the ions of the crystal and the solvation energy so released is sufficient to overcome the attraction between the ions in the crystal lattice.
Non-polar solvents like benzene (C6H6) and carbon tetrachloride (CCl4) does not solvate the ions as their dielectric constant is very low and thus they does not dissolved in ionic compounds.
Ionic compounds like sulphate and phosphate of barium and strontium are insoluble in water. This can be attributed to the high lattice energies of these compoundes due to polyvalent nature (high charges) of both the cations and anions. In these cases solvation energy is not sufficient to break the crystal lattice in these compounds.
Ex.10 Why are ionic compound solids have high density and high melting point ?
Sol. An ionic compounds is formed when a positively charged ion attracts a negatively charged ion. It does not ends with the attraction of a pair of oppositely charged particles, but a positively charged particle/ion can attracts as many negative charges as it can. Likewise a negatively charged ion attracts positively charged ion. The result is that positively and negatively charged ions are systematically arranged in three dimensions so that the final material has no nett charge. Such a regular arrangement is called lattice.
It is seen from the figure given below that the oppositely charged ions are arranged in a particular pattern. The arrangement of sodium chloride lattice is such that each Na+ ion is surrounded by six Cl– ions and each Cl– ion is surrounded by six Na+ ions
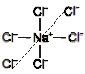
This kind of an arrangement is called electrovalent compounds due to which they have high density and high melting point.
Ex.11 How is an ore different from a mineral ?
Sol. The metals found in nature in combined state are called minerals. The minerals from which the metals can be profitably and economically extracted is called ore.
Ex.12 Discuss with examples the various types of ores from which metals are extracted ?
Sol. Ores are classified into the following types :
(i) Native ores. Silver, gold, platinum etc. are found in native state in the earth’s crust.
(ii) Oxide ores. An ore in which a metal occurs as an oxide is called an oxide ore. For example bauxite (Al2O3 .2H2O), haematite (Fe2O3) ; pyrolusite (MnO2)
(iii) Sulphide ores. An ore in which a metal occurs as sulphide is called a sulphide ore. For example, Iron pyrites (FeS), Galena (PbS), Copper pyrites (CuFeS2), Cinnabar (HgS).
(iv) Carbonate ores. An ore in which a metal occures as carbonate or basic carbonate is called a
carbonate ore. For example, limestone (CaCO3), dolomite (CaCO3, MgCO3)
(v) Halide ores. Aluminium occurs as a fluoride, e.g., cryolite (Na3AlF6), Sodium and potassium occur as chloride, e.g., carnallite (KCl, MgCl2 .6H2O), rocks salt (NaCl).
(vi) Sulphate ores. Barium and lead occur as barytes (BaSO4) and anglesite (PbSO4) respectively
Ex.13 What do you understand by the following terms :
(i) acidic flux (ii) basic flux (iii) slag.
Or
What is the differences between flux and slag ?
Sol. (i) Acidic flux. SiO2 is an acidic flux. It is used to remove basic impurities such as lime (CaO) from the ore.
![]()
(ii) Basic flux. Limestone (CaCO3) and magnesite (MgCo3) are examples of basic flux. These can be used to remove acidic impurities such as SiO2.
![]()
(iii) Slag. Some substances when heated with the ore combine with the earthy impurities and form easily fusible mass. The easily fusible mass is called slag which is lighter than molten metal and can be removed from the surface of the molten metal.
Ex.14 Give a chemical method of separating impurities from ores.
Or
Explain the term Leaching giving an example.
Sol. The chemical method of concentrating an ore is called leaching. It is a process in which soluble components of the ore are washed or extracted from insoluble materials by treating it with a chemical.
Example : naturally occurring ore is aluminium bauxits (AI2O3 . 2H2O) contains ferric oxide, silica and titanium dioxide as impurities. The powdered ore is digested with caustic soda solution (NaOH) under pressure for several hours. Aluminium oxide dissolves as aluminate while impurities are unaffected and remain as suspended material which are removed by filtration. The solution containing aluminium is diluted and agitated whereby aluminium is precipitated as aluminium hydroxide which is then heated to get pure alumina.
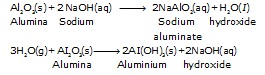
Ex.15 Why is extraction of metal always a reduction process ?
Sol. Because a metal in the combined state has positive valency and a positive ion gets converted into atom or metal on addition of electron(s). Thus, by definition it is a reduction process.
![]()
Ex.16 Distinguish between alloy and amalgam ?
Sol. An alloy is a homogeneous solid solution of a metal with other metals or non-metals, with essentially metallic properties.
An amalgam is an alloy of a metal with the mercury.
Ex.17 What is corrosion. Explain giving an example.
Sol. When the surface of a metal is attacked by air, water and some other substance, it is said to corrode. The phenomenon is known as corrosion.
When iron is exposed to moist air for a long time, its surface acquires a coating of a brown, flaky substance. The brown surface easily peel off the iron surface, which if exposed further to moist air again acquires more brown layer. This is due to corrosion of iron in moist air. The flaky substance formed is called rust. Rust is mainly hydrated ferric oxide, Fe2O3.xH2O.
Another, example of copper metal. The surface of copper in moist air acquires a green coating of basic copper carbonate, Cu(OH)2.CuCO3.
Ex.18 Explain the meaning of malleable and ductile.
Sol. Malleable means that metals can be beaten into thin sheets with a hammer (with out breaking). For example, if we take a piece of Al metal and beat it with a hammer four or five times, we will find that the piece of Al metal turns into a thin Aluminium sheet, without breaking. Ductile means that metals can be drawn (or stretched) into thin wire. For example, gold is the most ductile metal. Just 1 gm of Gold (Au) can be drawn into a very thin wire about 2 kilometres long.
Ex.19 Why is titanium called a strategic metal? Mention two of its properties which make it so special.
Sol. Titanium is called strategic metal because it is used for making certain war equipments. The properties which make the metal so special are:
(i) It is light in weight but at the same time stronger than the other metals.
(ii) it is not affected by corrosion even if kept in the open for a very long time.
Ex.20 An athlete won a bronze medal in a race competition. After some days, he found that the medal had lost its lustre due to the formation of a greenish layer on it. Name the metals present in the medal. What is the reason for the appearance of a greenish layer on its surface?
Sol. The bronze medal is an alloy and the constituting metals are copper and tin. The loss of lustre by the medal is due to the formation of a coating of green layer. This layer is of basic copper carbonate.
Ex.21 Arrange the following metals in decreasing order of their reactivity:
(i) Cu, Ca, Mg, Na, Zn.
(ii) Which metal listed in (i) is most likely to occur in the native state ?
Sol. (i) Based on the activity series, the decreasing order of reactivity of metals is:
Na > Ca > Mg > Zn > Cu
(ii) Copper is most likely to occur in the native (or free) state to a very small event.
Ex.22 Give reasons of the following:
For making gold ornaments, 22-carat gold is generally preferred to 24-carat gold
Sol. 24-carat gold is quite pure and is veryb soft. As such, it can not be used for making gold ornaments
22-carat gold is an alloy of gold containing a small amount of copper or silver. It is hard and more ductile as compared to pure gold. The ornaments are generally made from 22-carat gold.
Ex.23 (a) Are all pure liquids bad conductors of electricity?
(b) Name a liquid which is a good conductor of electricity but does not undergo electrolysis on passing electric current.
(c) If pure water is used, no electrolysis takes place. Why?
(d) Name one practical application based on the phenomenon of electrolysis.
Sol. (a) No, there are exceptions also. Mercury in pure state is a good conductor of electricity.
(b) Mercury is a good conductor of electricity but does not undergo electrolysis.
(c) Pure water (H2O) does not dissociate itself on passing electric current.
(d) The process of electroplating on the surface of metals is based on the phenomenon of electrolysis.
Ex.24 (a) Why are ionic compound usually hard?
(b) Why ionic compounds in the solid state does not conduct electricity and does the same in the molten state?
Sol. (a) Ionic compounds are very closely packed in space. As a resut, the vacant spaces or sites are quite less and the attrractive forces among the ions are very strong. They are therefore, generally hard.
(b) The conductivity of ionic compound is due to the momentum or mobility of the ions that are present. For example, the electrical conductivity of sodium chloride (Na+ Cl–) is due to of the mobility of the ions present. Since the ions can move only in the molten state and not in the solid state, these compounds are conducting only in the molten state.
Ex.25 Alloys are used in electrically heating devices rather than pure metals. Give one reason.
Ans. Alloys are generally the combination of two or more metals. Since metals are good conductors of electricity, a combination of metals i.e. alloy is expected to be a better conductor of electricity than the pure metal.
Q.1 Give an example of a metal which
(i) is a liquid at room temperature.
(ii) can be easily cut with a knife.
(iii) is the best conductor of heat.
(iv) is a poor conductor of heat.
Ans. (i) Metal that exists in liquid state at room temperature -Mercury
(ii) Metal that can be easily cut with a knife -Sodium
(iii) Metal that is the best conductor of heat -Silver
(iv) Metals that are poor conductors of heat Mercury and iead.
Q.2 Explain the meanings of malleable and ductile.
Ans. Malleable: Substances that can be beaten into thin sheets are called malleable. For example, most of the metals are malleabe.
Ductile: Substances that can be drawn into thin wires are called ductile. For example, most of the metals are ductile.
Q.3 Why sodium is kept immersed in kerosene oil ?
Ans. Sodium and potassium are very reactive metals and combine explosively with air as well as water. Hence, they catch fire if kept in open. Therefore, to prevent accidental fires and accidents, sodium is stored immersed in kerosene oil.
Q.4 Write equations for the reactions of
(i) Iron with steam
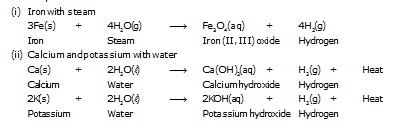
Q.5 Samples of four metals A, B, C and D were taken and added to the following solutions one by one. The results obtained have been tabulated as follows.
Here N.R. = No reaction, Dis. = Displacement Use the above table to answer the following questions about metals A, B, C and D.
(i) Which is the most reactive metal?
(ii) What would you observe if B is added to a solution of copper (II) sulphate?
(iii) Arrange the metals A, B, C and D in the order of decreasing reactivity.
Ans. A + FeSO4 ¾® No reaction, i.e., A is less reactive than iron
A + CuSO4 ¾® Displacement, i.e., A is more reactive than copper
B + FeSO4 ¾® Displacement, i.e., B is more reactive than iron
B + ZnSO4 ¾® No reaction, i.e., B is less reactive than zinc
C + CuSO4 ¾® No reaction, i.e., C is less reactive than iron
C + ZnSO4 ¾® No reaction, i.e., C is less reactive than zinc
C + AgNO3 ¾® Displacement, i.e., C is more reactive than silver
D + FeSO4/CuSO4/ZnSO4/AgNO3 ¾® No reaction, i.e., D is less reactive than iron, copper, zinc and silver.
From the above equations, we obtain:
(i) B is the most reactive metal.
(ii) If B is added to a solution of copper (II) sulphate, then it would displace copper.
B + CuSO4 –Displacement
(iii) The arrangement of the metals in the order of decreasing reactivity is:
B > A > C > D
Q.6 Which gas is produced when dilute hydrochloric acid is added to a reactive metal? Write the chemical reaction when iron reacts with dilute H2SO4.
Ans. Hydrogen gas is evolved when dilute hydrochloric acid is added to a reactive metal.
When iron reacts with dilute H2SO4, iron (II) sulphate with the evolution of hydrogen gas is formed.
![]()
Q.7 What would you observe when zinc is added to a solution of iron (II) sulphate? Write the chemical reaction that takes place.
Ans. Zinc is more reactive than iron. Therefore, if zinc is added to a solution of iron (II) sulphate, then It would displace iron from the solution.
![]()
Q.8 (i) Write the electron-dot structures for sodium, oxygen and magnesium.
(ii) Show the formation of Na2O and MgO by the transfer of electrons.
(iii) What are the ions present in these compounds?
Ans. (i) The representation of elements with valence electrons as dots around the elements is referred to as electron-dot structure for elements.
![]()
(c) Magnesium (2, 8, 2) =
(ii) 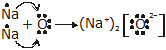
(iii) The ions present in Na2O are Na+ and O2– ions and in MgO are Mg2+ and O2– ions.
Q.9 Why do ionic compounds have high melting points?
Ans. Ionic compounds have strong electrostatic forces of attraction between the ions. Therefore, it
requires a lot of energy to overcome these forces. That is why ionic compounds have high melting points.
Q.10 Define the following terms.
(i) Mineral (ii) Ore (iii) Gangue
Ans. (i) Mineral: Most of the elements occur in nature in combined state as minerals. The chemical composition of minerals is fixed.
(ii) Ore : Minerals from which metals can be extracted profitably are known as ores.
(iii) Gangue: The impurities (sand, silt, soil, gravel, etc.) present in the ore are called gangue.
Q.11 Name two metals which are found in nature in the free state.
Ans. The metals at the bottom of the reactivity series are mostly found in free state. For example: gold, silver, and platinum.
12. What chemical process is used for obtaining a metal from its oxide ?
Ans. The chemical process used for obtaining a metal from its oxide is reduction. In this process, metal oxides are reduced by using Suitable reducing agents such as carbon or by using highly reactive metals to displace the metals from their oxides.
For example, zinc oxide is reduced to metallic zinc by heating with carbon.
ZnO(s) + C(s) Zn(s) + CO(g)
Manganese dioxide is reduced to manganese by treating it with aluminium powder. In this case, aluminium displaces manganese from its oxide.
3MnO2(g) + 4Al(s) ¾® 3Mn(l) + 2Al2O3(s) + Heat
Oxides of more reactive metals are reduced by electrolysis.
Q.13 Metallic oxides of zinc, magnesium and copper were heated with the following metals.
In which cases will you find displacement reactions taking place?
Ans.
Here N.R. = No reaction, Dis. = Displacement.
Q.15 Which metals do not corrode easily?
Ans. More reactive a metal is, more likely it is to be corroded. Therefore, less reactive metals are less likely to get corroded. this is why gold plating provides high resistance to corrosion.
Q.16 What are alloys?
Ans. Alloys are homogeneous mixtrues of two or more elements. The elements could be two metals, or a metal and a non-metal. Any alloy is formed by first melting the metal and then dissolving the other elements in it. For example, steel is an alloy of iron and carbon.
Q.17 Which of hte following pairs will give displacement reactions?
(a) NaCl solution and copper metal
(b) MgCl2 solution and aluminium metal
(c) FeSO4 solution and silver metal
(d) AgNO3 solution and copper metal
Ans. (d) AgNO3 solution and copper metal
Q.18 Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) all of the above.
Ans. (c) Applying a coating of zinc. One can also apply grease and paint to prevent iron from rusting. However, in case of iron frying pan, grease and paint cannot be applied because when the pan will be heated and washed again and again, the coating of grease and paint would get destroyed.
Q.19 An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) Calcium (b) Carbon (c) Silicon (d) Iron
Ans. (a) The element is likely to be calcium
Q.20 Food cans are coated with tin and not with zinc because
(a) zinc is costlier than tin.
(b) zinc has a higher melting point than tin.
(c) zinc is more reactive than tin.
(d) zinc is less reactive than tin.
Ans. (c) Food cans are coated with tin and not with zinc because zinc is more reactive than tin.
Q.21 You are given a hammer, a battery, a bulb, wires and a switch.
(a) How could you use them to distinguish between samples of metals and non-metals?
(b) Assess the usefulness of these tests in distinguishing between metals and non-metals.
Ans. (a) With the hammer, we can beat the sample and if it can be beaten into thin sheets (that is, it is malleable), then it is a metal otherwise a non metal. Similarly, we can use the battery, bulb, wires, and a switch to set up a circuit with the sample. If the sample conducts electricity, then it is a metal otherwise a non-metal.
(b) The above tests are useful in distinguishing between metals and non-metals as these are based on the physical properties. No chemical reactions are involved in these tests.
Q.22 What are amphoteric oxides? Give two examples of amphoteric oxides.
Ans. Those oxides that behave as both acidic and basic oxides are called amphoteric oxides.
Examples: aluminium oxide (Al2O3), zinc oxide (ZnO)
Q.23 Name two metals which will displace hydrogen from dilute acids, and two metals which will not.
Ans. Metals that are more reactive than hydrogen displace it from dilute acids. For example: sodium and potassium. Metals that are less reactive than hydrogen do not displace it. For example: copper and silver.
Q.24 In the electrolytic refining of a metal M. what would you take as the anode, the cathode and the electrolyte ?
In the electrolytic refining of a metal M:
Ans. Anode -Impure metal M
Cathode -Thin strip of pure metal M
Electrolyte -Solution of salt of the metal M
Q.25 Pratyush took sulphur powder on a spatula and heated it. He collected the gas evolved by inverting a test tube over it, as shown in figure below.
(a) What will be the action of gas on
(i) dry litmus paper?
(ii) moist litmus paper?
(b) Write a balanced chemical equation for the reaction taking place.
Ans. (a) (i) There will be no action on dry litmus paper.
(ii) Since the gas is sulphur dioxide (SO2), it turns moist blue litmus paper to red because sulphur dioxide reacts with moisture to form sulphurous acid.
![]()
Q.26 State two ways to prevent the rusting of iron. Two ways to prevent the rusting of iron are:
Ans. (i) Oiling, greasing, or painting: By applying oil, grease, or paint, the surface becomes water proof and the moisture and oxygen present in the air cannot come into direct contact with iron. Hence, rusting is prevented.
(ii) Galvanisation : An iron article is coated with a layer of zinc metal, which prevents the iron to come in contact with oxygen and moisture. Hence, rusting is prevented.
Q.27 What type of oxides is formed when non-metals combine with oxygen?
Ans. Non-metals combine with oxygen to form acidic oxides.
For examples:
![]()
Q.28 Give reasons.
(a) Platinum, gold and silver are used to make jewellery.
(b) Sodium, potassium and lithium are stored under oil.
(c) Aluminium is a highly reactive metal, yet it is used to make utensils for cooking.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Ans. (a) Platinum, gold, and silver are used to make jewellery because they are very lustrous. Also, they are very less reactive and do not corrode easily.
(b) Sodium, potassium, and lithium are very reactive metals and react very vigorously with air as well as water. Therefore, they are kept immersed in kerosene oil in order to prevent their contact with air and moisture.
(c) Though aluminium is a highly reactive metal, it is resistant to corrosion. This is because aluminium reacts with oxygen present in air to form a thin layer of aluminium oxide. This oxide layer is very stabie and prevents further reaction of aluminium with oxygen. Also, it is light in weight and a good conductor of heat. Hence, it is used to make cooking utensils.
(d) Carbonate and sulphide ores are usually converted into oxides during the process of extraction because metals can be easily extracted from their oxides rather than from their carbonates and sulphides.
Q.29 You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Ans. Copper reacts with moist carbon dioxide in air to form copper carbonate and as a result, copper vessel loses its shiny brown surface forming a green layer of copper carbonate. The citric acid present in the lemon or tamarind neutralises the basic copper carbonate and dissolves the layer. That is why, tarnished copper vessels are cleaned with lemon or tamarind juice to give the surface of the copper vessel its characteristic lustre.
Q.30 Differentiate betv.;een metal and non-metal on the basis of their chemical properties.
Ans. Metals:
(i) Metals are electropositive.
(ii) They react with oxygen to form basic oxides.
(iii) These have ionic bonds.
(iv) They react with water to form oxides and hydroxides. Some metals react with cold water, some with hot water, and some with steam.
(v) They react with dilute acids to form a salt and evolve hydrogen gas. However, Cu, Ag, Au, Pt, Hg do not react.
(vi) They react with the salt solution of metals. Depending on their reactivity. displacement, reaction can occur.
(vii)These act as oxidising agents (as they can gain electrons).
Non-metals:
(i) Non-metals are electronegative.
(ii) They react with oxygen to form acidic or neutral oxides.
(iii) These have covalent bonds.
(iv) They do not react with water.
(v) They do not react with dilute acids. These are not capable of replacing hydrogen.
(vi) These react with the salt solution of non-metals.
(vii)They act as reducing agents (as they can easily lose electrons).
Q.31 A man went door to door posing as a goldsmith, He promised to bring back the glitter of old and dull gold ornaments. An unsuspecting lady gave a se: of gold bangles to him which he dipped in a particular solution, The bangles sparkled like new but their weight was reduced drastically. The lady was upset but after a futile argument the man beat a hasty retreat. Can you play the detective to find out the nature of the solution he had used?
Ans. He must have dipped the gold metal in the solution of aqua regia -a 3:1 mixture of cone. HCl and conc. HNO3. Aqua regia is a fuming, highly corrosive liquid. It dissolves gold in it. After dipping the gold ornaments in aqua regia. the outer layer of gold gets dissolved and the inner shiny layer appears. That is why the weight of gold ornament reduced.
Q.32 Give reasons why copper is used to make hot water tanks and not steel (an alloy of iron).
Ans. Copper does not react with cold water, hot water, or steam. However, iron reacts with steam. If the hot water tanks are made of steel (an alloy of iron), then iron would react vigorously with the steam formed from hot water.
![]()
That is why copper is used to make hot water tanks, and not steel.
Q.1 Define metals.
Q.2 Name a metal which can be cut with knife.
Q.3 Name two metals which does not react with oxygen.
Q.4 What type of oxides are formed when metals combine with oxygen.
Q.5 Name two metals which are found in nature in free state.
Q.6 Give electron dot structure of chlorine and oxygen.
Q.7 Explain why the surfaces of some metals acquire dull appearance when exposed to air for long period of time.
Q.8 State two ways to prevent rusting of iron.
Q.9 What are amphoteric oxides? Give two examples of amphoteric oxides?
Q.10 Define alloy. Give two advantage of making alloys.
Q.11 Give three difference between metals and non-metals based on physical properties.
Q.12 Give reasons.
1. Aluminium oxide is amphoteric oxide
2. Non-metals does not conduct electricity
3. Metals displace hydrogen gas from acids
Q.13 Explain why?
(a) Iron articles are frequently painted
(b) Iron sheets are coated with zinc layer.
Q.14 What is activity series of metals, Arrange the given metals in activity series: Fe, Au, Zn, Al, Cu.
Q.15 What determines the reactivity of metals ?
Q.16 What happen when :
(i) Lead is heated to 400ºC–500°C in air.
(ii) Steam is passed over heated iron.
(iii) Copper oxide is heated with magnesium. (iv) Aluminium wire is dipped in heating water.
Q.17 How would you show that silver is chemically less reactive than copper ?
Q.18 Name two metals which may occur as sulphides.
Q.19 Name a metal which occurs as fluoride.
Q.20 Name two metals which may occur as carbonates.
Q.21 Identify the most reactive and the least reactive metal amongst the following :
Al, K, Cu, Au
Q.22 An oxide ore has been found to contain some impurities which are magnetic. State the process to concentrate this ore.
Q.23 For reduction of metallic oxide (M2O3) suggest a reducing agent cheaper than aluminium ?
Q.24 Name the process in relation to metallurgy whereby an ore is heated strongly in absence of air.
Q.25 Name the process in relation to metallurgy whereby an ore is heated in excess of air or oxygen.
Q.26 What types of ores are treated in ‘calcination’
Q.27 A light and strong alloy is needed for making instruments. What metal should be added to a small amount of magnesium to obtain the alloy
Q.28 Give reason why copper is used to make hot water tanks but steel (an alloy of iron) is not.
Q.29 Name an ore of zinc other than zinc oxide. By what process can this ore be converted to zinc oxide ?
Q.30 Select the metalloids/ from amongst the following elements.
(i) Bismuth (ii) Copper
(iii) Zinc (iv) Iron
Q.31 What type of reaction is involved in rusting ?
Q.32 From amongst the metals sodium, calcium, aluminium, copper and magnesium, name the metal
(i) which reacts with water only on boiling and
(ii) another which does not react even with steam.
Q.33 An element reacts with oxygen to form an oxide which dissolves in dilute hydrochloric acid. The oxide also turns a solution of red litmus blue. Is the element a metal or a non-metal ? Explain with the help of a suitable example.
Q.34 Choose the metal (from the list given below) which can displace zinc from zinc sulphate solution. Lead, Copper, Magnesium, Silver. Write the equation of the chemical reaction involved.
Q.35 Explain why sodium is not found in the native state.
Q.1 Which is the least conductor of heat –
(A) Gold (B) Platinum
(C) Silver (D) Lead
Q.2 Metal have ............no. of electrons in their outer most shell –
(A) 1 to 8 (B) 7 to 9
(C) 1 to 3 (D) 10 to 12
Q.3 Which oxide is neutral ?
(A) NO2 (B) MgO
(C) H2O (D) None of these
Q.4 Al2.O3 2SiO2.2H2O is the chemical formula of–
(A) Bauxite (B) Haemetite
(C) China Clay (D) Monazite
Q.5 An alloy is –
(A) A element (B) A mixture
(C) An isomer (D) A metalloid
Q.6 Which non-metal is the best conductor of electricity –
(A) Phosphorus (B) Fluorine
(C) Graphite (D) Bromine
Q.7 Which compound is used in photography –
(A) AgNO3 (B) AgO
(C) AgBr (D) AgCl
Q.8 Carnallite is the mineral of –
(A) Na (B) Ca
(C) Mg (D) All of these
Q.9 The magnesium is used in –
(A) Flash bulb (B) Grignard reagent
(C) Electron alloy (D) All of these
Q.10 Cinnabar is an ore of –
(A) Mercury (B) Copper
(C) Calcium (D) Lead
Q.11 The constituent of haemoglobin is –
(A) Iron (B) Sodium
(C) Copper (D) Magnesium
Q.12 The most abundant metal in the earth crust is –
(A) Al (B) Fe
(C) O (D) Cu
Q.13 Because of high electropositively, the atom of metals can easily form –
(A) Positive ions (B) Negatively ions (C) Neutral ions (D) Covalent bonds
Q.14 Volatile metals are purified by –
(A) Oxidation (B) Distillation
(C) Liquation (D) Electrolytic refining
Q.15 Amalgam is the homogeneous mixture of –
(A) Metal and metal
(B) Metal and mercury
(C) Metal and non-metal
(D) All of these
Q.16 Which of the following is a ferrous alloy–
(A) Solder (B) Brass
(C) Magnalium (D) Steel
Q.17 Which of the following statements is correct–
(A) All minerals are ores
(B) All ores are minerals
(C) Some ores are minerals
(D) None is correct
Q.18 Food cans are coated with tin and not zinc because –
(A) Zinc is costlier than tin
(B) Zinc has a higher melting point than tin (C) Zinc is more reactive than tin (D) Zinc is less reactive than tin
Q.19 Elements with atomic number greater than
92 are also called_____ [NTSE]
(A) alkali metals
(B) alkaline earth metals
(C) transuranic elements
(D) noble gas elements
Q.20 A purple-coloured solid halogen is __.[NTSE]
(A) CI (B) Br
(C) I (D) F
Q.21 Chajcogen refers to ______. [NTSE]
(A) alkali-forming elements
(B) salt-forming elements
(C) ore-forming elements
(D) chemically inert elements
Q.22 Which ofthe following is a solid at STP?[NTSE]
(A) fluorine (B) chlorine
(C) bromine (D) iodine
Q.23 Element M is a metal and its chloride has the formula MCl2. The element most likely belongs to which group of the periodIC table'?[NTSE]
(A) 1 (B) 2
(C) 15 (4) 17
Q.24 Copper is extracted from low grade ore by _____. [NTSE]
(A) hydrometallurgy
(B) pyrometallurgy
(C) electrometallurgy
(D) all of the above processes
Q.25 Roasting process is applied to which of the following ores? [NTSE]
(A) galena (B) iron pyrites
(C) copper glance (D) all of these
Q.26 Purification of metals by liquation method is based on difference in ______. [NTSE]
(A) density (B) melting point
(C) solubility (D) vapour density
Q.27 Arrange the following metal in the increasing order of their reactivity towards water: Zinc, Iron, Magnesium, Sodium. [NTSE]
(A) iron < Magnesium < sodium < zinc (B) iron < zinc < magnesium < sodium
(C) magnesium < iron < sodium < zinc (D) sodium < iron < magnesium < zinc
Q.28 Which of the following is used for making magnets? [NTSE]
(A) duralumin (B) magnalium
(C) bronze (D) alnico
Q.29 White phsphorus is stored in [NTSE]
(A) ether (B) water
(C) alcohol (D) kerosene oil
Q.30 Ordinary glass is a mixture of [NTSE]
(A) sodium silicate, calcium silicate (B) sodium silicate, calcium silicate and silica
(C) sodium silicate and silica
(D) none of the above
Q.31 Annealing is done to [NTSE]
(A) increase brittleness
(B) increase Transparancy
(C) decrease brittleness
(D) increase refractive inded
Q.32 Polymer used in making floor tiles is [NTSE]
(A) teflon (B) polypropylene
(C) polyyinyl chloride (D) buta-1, 3-diene
Q.33 Which of the following is an example of condensaiton polymers? [NTSE]
(A) polyethene (B) neoprene
(C) teflon (D) nylon
Q.34 Insulation of electric wire is done by[NTSE]
(A) isoprene (B) neoprene
(C) vinyl chloride (D) buta-1, 3-diene
Q.35 Permanent magnets can be made from[NTSE]
(A) Ni steel (B) Cobalt steel
(C) Stainless steel (D) Wrought iron
Q.36 The formula of Oleum is [NTSE]
(A) H2SO4 (B) H2S2O7
(C) H2S2O3 (D) H2S2O6
Q.37 The main constituents of cement are:[NTSE]
(A) calcium oxide, silicon dioxide, Aluminium oxide
(B) calcium oxide, Iron oxide, Sulphur dioxide
(C) magnesium oxide, Silicon dioxide, Aluminium oxide
(D) none of these
Q.38 Hard glass is prepared by [NTSE]
(A) fusing a mixutre of sodium carbonate, calcium carbonate and silica
(B) fusing a mixture of potassium carbonate, calcium carbonate and silica
(C) fusing a mixture of potassium carbonate and any oxide
(D) none of the above
Q.39 The soap can be hardened by [NTSE]
(A) adding sodium carbonate or sodium silicate during its manufacture
(B) adding sodium chloride
(C) adding potassium hydroxide
(D) adding animal fat and coconut oil
Q.40 Soda Acid for extinguisher contains [NTSE]
(A) sodium carbonate and nitric acid (B) sodium hydrogen carboante and sulphuric acid
(C) sodium carboante and carbonic acid (D) sodium chloride and sulphuric acid
Q.41 The real bleaching agent present in bleaching powder is [NTSE]
(A) chlorine
(B) oxygen
(C) CaO
(D) none of the above
Q.42 Cake does not taste bitter due to presence of [NTSE]
(A) sodium carbonate
(B) tartaric acid
(C) citric acid
(D) sugar
Q.43 What is obtained on adding lime to H2O?
[NTSE]
(A) lime
(B) limestone
(C) slaked lime
(D) quicklime
1. D 2. C 3. C 4. C
5. B 6. C 7. C 8. C
9. D 10. A 11. A 12. A
13. A 14. B 15. B 16. D
17. A 18. C 19. C 20. C
21. C 22. D 23. B 24. A
25. D 26. B 27. B 28. D
29. B 30. A 31. C 32. C
33. D 34. C 35. D 36. B
37. A 38. B 39. A 40. B
41. A 42. B 43. C
1. Which of the following properties is not associated with metals?
(A) Allotropy (B) Conductivity (C) Ductility (D) Malleability
2. Which of the following elements is a metal?
(A) Aluminium (B) Boron (C) Carbon (D) Silicon
3. Which of the following elements is not a metal?
(A) Calcium (B) Copper (C) Potassium (D) Sulphur
4. Which of the following elements is a metalloid?
(A) Carbon (B) Arsenic (C) Beryllium (D) Sulphur
5. Iron is a/an :
(A) Alloy (B) Metal (C) Metalloid (D) Nonmetal
6. Graphite is a/an :
(A) Alloy (B) Metal (C) Metalloid (D) Nonmetal
7. Stainless steel is a/an :
(A) Alloy (B) Metal (C) Metalloid (D) Nonmetal
8. Which of the following nonmetals is a good conductor of electricity?
(A) Diamond (B) Graphite (C) Phosphorus (D) Sulphur
9. The reactivities of iron, magnesium, sodium and zinc towards water are in the following order:
(A) Fe > Mg > Na > Zn (B) Zn > Na > Mg > Fe (C) Na > Mg > Zn > Fe (D) Mg > Na > Fe > Zn
10. Hydrogen is not a metal but it has been included in the reactivity series because it :
(A) Forms positive ions like metals (B) Is a diatomic
(C) Is the first element of the periodic table (D) Reacts with metals
11. Which of the following metals constitutes the alloy magnalium?
(A) Al, Cu (B) Al, Fe (C) Ag, Mg (D) Al, Mn
12. One of the constituents of amalgam is :
(A) Aluminium (B) Copper (C) Iron (D) Mercury
13. Which of the following metals reacts with water causing fire?
(A) Aluminium (B) Calcium (C) Sodium (D) Zinc
14. Which of the following metals is used in storage battery?
(A) Iron (B) Lead (C) Tin (D) Zinc
15. Which of the following alloys does not contain copper?
(A) Bell metal (B) Brass (C) Bronze (D) Solder
16. Which of the following metals reacts with water/steam to produce oxide instead of hydroxide?
(A) Sodium (B) Potassium (C) Calcium (D) Magnesium
17. Which of the following metals does not react with dilute hydrochloric acid producing hydrogen gas?
(A) Zinc (B) Tin (C) Lead (D) Copper
18. Which of the following alloys does not contain tin?
(A) Bronze (B) Gun metal (C) German silver (D) Type metal
19. The metal which exists as a liquid is :
(A) Aluminium (B) Sodium (C) Mercury (D) Potassium
20. Which of the following nonmetals exists in the liquid form?
(A) Chlorine (B) Bromine (C) Iodine (D) Fluorine
21. Which of the following nonmetals exists in the solid phase?
(A) Fluorine (B) Chlorine (C) Bromine (D) Iodine
22. Nonmetals do not conduct electricity. However an allotrope of a nonmetal is used as electrode. It is :
(A) Diamond (B) Graphite (C) Sulphur (D) Phosphorus
23. The hardest nonmetal amongst sulphur, graphite, diamond and silicon is :
(A) Sulphur (B) Graphite (C) Diamond (D) Silicon
24. Which of the following nonmetals sublimes on heating?
(A) Fluorine (B) Chlorine (C) Bromine (D) Iodine
25. Which of the following elements produces acidic oxide on reacting with oxygen?
(A) Sodium (B) Chlorine (C) Magnesium (D) Zinc
26. Which of the following elements produces basic oxide on reacting with oxygen?
(A) Chlorine (B) Sulphur (C) Phosphorus (D) Magnesium
27. Which of the following replacement reactions is not possible?
![]()
28. Copper sulphate solution can be safely kept in a container made of :
(A) Aluminium (B) Lead (C) Silver (D) Zinc
29. Most metals can be drawn into thin wires. This property is known as :
(A) Allotropy (B) Conductivity (C) Ductility (D) Malleability
30. Most metals can be drawn into sheets. This property is knwon as :
(A) Allotropy (B) Conductivity (C) Ductility (D) Malleability
31. Which of the following oxides is neither acidic nor basic?
(A) CO2 (B) N2O (C) SO2 (D) SO3
32. Which of the following oxides may be said to be neutral oxide?
(A) CO (B) CO2 (C) SO2 (D) SO3
33. Which of the following elements does not react with cold acids?
(A) Na (B) Mg (C) C (D) Zn
34. Which of the following elements would produce covalent hydride?
(A) Na (B) Mg (C) Cl (D) K
35. Which of the following nonmetal is used in polymers?
(A) Nitrogen (B) Phosphorus (C) Silicon (D) Sulphur
36. The second most abundant element in the earth's crust is :
(A) Oxygen (B) Silicon (C) Aluminium (D) Iron
37. The first most abundant element in the earth's crust is :
(A) Oxygen (B) Silicon (C) Aluminium (D) Iron
38. The element silicon exists as :
(A) Gas (B) Liquid (C) Soft solid (D) Hard solid
39. The element silicon exists as :
(A) Crystalline covalent solid (B) Crystalline ionic acid
(C) Amorphous solid (D) Liquid
40. Silicon does not react with acids except
(A) Hydrochloric acid (B) Hydrobromic acid (C) Hydroiodic acid (D) Hydofluoric acid
41. The elemental phosphorus is written as :
(A) P (B) P2 (C) P3 (D) P4
42. The white phosphorus is stored :
(A) In air (B) Under water (C) Under kerosene (D) Under CS2
43. Which of the following statements is true?
(A) Both white and red phosphorus are soluble in water
(B) Both white and red phosphorus are soluble in carbon disulphide
(C) White phosphorus is soluble in carbon disulphide whereas red phosphorus is insoluble
(D) White phosphorus is insoluble in carbon disulphide whereas red phosphorus is soluble
44. Which of the following statement is true?
(A) Both white and red phosphorus are reactive
(B) Both white and red phosphorus are inactive
(C) White phosphorus is reactive whereas red phosphorus is inactive
(D) White phosphorus is inactive whereas red phosphorus is reactive
45. Which of the following statements about phosphours is not true?
(A) Phosphorus does not occur in free state (B) Phosphorus is present in bones and teeth
(C) Phosphorus exists in several allotropic forms
(D) White phosphorus is much less active than red variety
46. When phosphorus is heated with conc. HNO3, it reduces the acid to :
(A) NO (B) NO2 (C) N2O3 (D) N2O5
47. When phosphorus is heated with conc. HNO3, it is oxidized to :
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
48. P4O6 is the anhydride of :
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
49. P4O10 is the anhydride of :
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
50. Which of the following is orthophosphorus acid?
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
51. Which of the following is orthophosphoric acid?
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
52. When P4O6 is dissolved in cold water, we obtain :
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
53. When P4O10 is boiled with water, the final product of hydrolysis is :
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
54. Which of the following is pyrophosphoric acid?
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
55. The elemental sulphur is written as :
(A) S (B) S2 (C) S4 (D) S8
56. Which of the following statements is true?
(A) Both rhombic and monoclinic sulphur are soluble in water
(B) Both rhombic and monoclinic sulphur are soluble in carbon disulphide
(C) Both rhombic and monoclinic sulphur are insoluble in carbon disulphide
(D) Rhombic sulphur can be converted into monoclinic sulphur but the reverse is not possible
57. Colloidal sulphur is obtained when :
(A) Sulphur is heated in absence of air (B) Sulphur is heated in presence of air
(C) Sodium thiosulphate is treated with iodine (D) H2S is passed through water containing HNO3
58. Which of the following forms of sulphur is stable at room temperature and 1 atmospheric pressure?
(A) Amorphous (B) Hexagonal (C) Monoclinic (D) Orthorhombic
59. Which of the following statement is not true of sulphur?
(A) Sulphur belongs to Group VIA of the periodic table
(B) Sulphur exhibits allotropy
(C) Sulphur occurs both in the native form and combined form
(D) Sulphur is soluble in water
60. Which of the following gases is used for the qualitative analysis of metal ions?
(A) CO2 (B) H2S (C) SO2 (D) SO3
61. Which of the following nitrogen oxide is neutral?
(A) N2O (B) NO2 (C) N2O3 (D) N2O5
62. Which of the following nitrogen oxides is produced during lighting bolts in the atmosphere?
(A) NO (B) NO2 (C) N2O3 (D) N2O5
63. Which of the following nitrogen oxides is an anhydride of HNO2?
(A) N2O (B) NO2 (C) N2O3 (D) N2O5
64. Which of the following nitrogen oxides is an anhydride of HNO3?
(A) N2O (B) NO2 (C) N2O3 (D) N2O5
65. Nitrates of all metals are :
(A) Coloured (B) Insoluble in water (C) Soluble in water (D) Unstable
66. Burning sulphur in air produces :
(A) H2S (B) H2S2 (C) SO2 (D) SO3
67. Sulphur dioxide is a strong reducing agent. However, it can also act as an oxidizing agent. Which of the following reactions shows its oxidizing nature?
(A) Bleaching flower petals (B) Decolourising of acidified KMnO4 solution
(C) Reaction with H2S to give sulphur (D) Turning acidified dichromate paper green
68. Which of the following is not true for SO2?
(A) SO2 is obtained by roasting metal sulphides (B) SO2 is acidic in nature
(C) SO2 is used as a disinfectant (D) SO2 is an anhydride of H2SO4
69. Which of the following is an anhydride of H2SO4?
(A) H2S (B) H2S2 (C) SO2 (D) SO3
70. Which of the following is an anhydride of H2SO3?
(A) H2S (B) H2S2 (C) SO2 (D) SO3