Heat : It is a form of energy which causes the sensation of hotness or coldness.
For example, if we dip our finger in hot water we have a sensation of hotness. Similarly, If we touch a block of ice the sensation is that of coldness. In the former case the heat energy has moved into the finger while in the later case it has moved out of the finger. Thus hotness or coldness basically indicates whether heat energy is flowing into our body or out of it.
Temperature : It is the effect of heat energy which determines the thermal state of a given substance. In other words it determines the degree of hotness or coldness of a substance. If a body is at a higher temperature than its surroundings, it means that heat energy will flow out of the body. Similarly, if a body is at a lower temperature than its surroundings, it means that heat energy will flow into the body.
Measurement of temperature: The instrument used for the measurement of temperature is called thermometer.
(A) Celsius or centigrade scale :
As the name suggests, this scale has 100 divisions between the upper and lower standard points. This scale was introduced by a Swedish astronomer Celsius and is known after his name. On this scale 0°C represent melting point of ice & 100°C represent steam point. Each division on this scale is called one degree centigrade or one degree Celsius and is written as °C.
(B) Fahrenheit Scale :
The scale was introduced by Fahrenheit. On this scale 32°F represents the melting point of 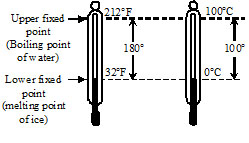
Relation between celsius and fahrenheit scales
(C) Kelvin Scale
The scale of measurement of temperature, in which lowest temperature is zero Kelvin (–273°C) is called Kelvin scale. Thus is also called S.I. scale of temperature.
Temp. in Kelvin = 273.15 + temp in °C
Characteristics of Kelvin scale
(i) There cannot be any temperature below zero Kelvin
(ii) Rise in temperature in Kelvin = Rise in temperature in degree Celsius.
ROLE OF TEMPERATURE IN TRANSFER OF HEAT ENERGY
When two bodies at different temperature are brought in contact with each other, the heat energy always flows from a body at higher temperature to a body at lower temperature, till the temperature equalise. Thus, it is the temperature of body which determines the direction of flow of heat energy.
Relation between different temperature
UNIT OF HEAT ENERGY
Heat energy is measured in calories.
The quantity of heat energy required to raise the temperature of 1 g of pure water through 1°C (14.5 °C to 15.5°C) is called one calories .
TRANSFER OF HEAT
Heat can be transferred by three methods-
(a) conduction
(b) Convection
(c) Radiation
(a) Conduction ;
Heat is transferred by the process of conduction when bodies at different temperatures are in direct or indirect physical contact
Conduction is a process of transfer of heat from the hotter end to the colder end from particle to particle of the medium. Conduction is the process of transmission of heat in solids, in which the molecules of the solid do not move from their position (only oscillate back and forth about their fixed positions) but merely transfer the heat energy in the form of kinetic energy from one molecule to the next.
Thus, medium is required for the transfer of heat by conduction, therefore, conduction is not possible in vacuum. In solids heat is transferred mainly by the process of conduction.
* Types of conductors
(i) Good conductors
The substances through which heat energy can easily flow by conduction are called good conductors.
Ex. Metals in general are good conductors. Amongst the metals, silver is considered best conductors. Amongst non-metal graphite is a good conductor. Metals are good conductor of heat
(ii) Bad conductors
The substances which do not allow the heat energy to flow through them easily are called poor conductors or bad conductors.
Ex. Amongst the solid, glass, wood, clay, asbestos, rubber, plastics, wax etc, are poor conductors, All liquids except mercury are poor conductors. All gases without any exception are poor conductors.
* Practical Application of good conductors
* Practical Application of bad conductors
We wear woolen clothes in the winter, because the woolen clothes contains a large amount of the trapped air. Since air is a bad conductor of heat, it does not allow the body heat to flow outward, As our body stops losing heat, we feel warm.
(b) Convection :
Convection is a process of transfer of heat by the actual movement of the medium particles. Liquids and gases are the bad conductor of heat. they are heated mainly by the process of convection. In a solid, the atoms cannot move, leaving their positions, So solids are not heated by convection. A medium is required for the transfer of heat by convection. Heat cannot be transferred by convection in vacuum.
(c) Radiation :
Radiation is the process of heat transfer in which heat directly passes from one body to the other body without affecting the medium. Thus, no medium is required for the heat transfer by the process of radiation. In vacuum, heat transfer takes place only by the process of radiation.
The heat energy transferred by the process of radiation is called the radiant heat or the thermal radiation.
(i) Nature of radiant heat : Heat energy is transferred by radiation in the form of electromagnetic waves. These waves can travel even in vacuum. They travel in all direction in straight line with a speed equal to the speed of light (= 3 × 108 ms–1). They do not heat the medium through which they pass. They are reflected by a polished and white surface. When radiant heat falls on an object, it is partially absorbed and partially reflected. Dull, black or coloured surfaces are good absorber and good radiators of heat.
(ii) Properties of heat radiations
(A) Heat radiations travel with the speed of light.
(B) Heat radiations can travel through vacuum
(C) Heat radiations travels in straight lines
(1).Heat is a form of energy. We use this energy to generate electricity, among other things.
(2).The hotness or coldness of a body is relative.
(3).The temperature of a body is a measure of the degree of hotness of the body. We measure it by comparing it with a universal standard.
(4).The Celsius and Fahrenheit scales are commonly used to measure temperature. Ice melts at 0ºC or 32ºF. and water boils at 100ºC or 212ºF.
(5).Heat causes a rise in temperature. It also causes expansion and change in the state of matter.
(6).Heat flows or travels in three ways – conduction, convection and radiation.
(7).Conduction is the process by which heat flows through a substance without the movement of the substance itself.
(8).Convection is the process of transfer of heat in a liquid or gas by the movement of the liquid or gas.
(9).Radiation s the process by which heat travels without the help of a material medium. Radiant heat travels in straight lines, in all direction from a hot body.
(10).Every body emits (gives out) radiant heat. The emission of radiant heat from a body depends upon its temperature and colour.
(11).When radiant heat falls on a body, a part of it is absorbed and the rest is reflected. How much radiant heat a body absorbs and how much it reflects depend on its colour and the nature of its surface. Shiny white bodies are the best reflectors, while rough black bodies are the best absorbers of radiant heat. Black bodies absorb and emit radiant heat the best.
Q.1 Explain with example to show that heat is a form of energy.
Q.2 What is temperature? Name the scales used to measure it.
Q.3 Distinguish between the Celsius and Fahrenheit scales.
Q.4 Mention three changes that heat causes in substances.
Q.5 A steel spoon dipped in a pot of boiling soup for a few minutes becomes too hot to touch. How does heat travel through the spoon?
Q.6 How is it that we can hold a match while it is burning?
Q.7 How does turning on a heater in a room make the whole room warm?
Q.8 How does heat from the sun reach the earth?
Q.9 Mention one similarity between heat and light.
Q.10 When two bodies are in contact, heat flows from the hotter to the colder body. When does heat stop flowing?
Q.11 If the hotness or coldness of a body is relative, how do we measure its degree of hotness?
Q.12 When a pan of water is heated on a gas stove, all the water turns hot in a while. How does heat travel through the water ?
Q.13 What happens to radiant heat when it falls on a body? What does the absorption of radiant heat by a body depend on?
Q.14 (a) Mention one difference between the transfer of heat by conduction and that by radiation.
(b) Mention one difference between the transfer of heat by conduction and that by convection.
Q.15 Explain how land and sea breezes originate.
Q.16 How would you demonstrate to a friend that gases expand when heated?
Q.17 Explain why -
(a) It is better to wear lighter shades in summer.
(b) Two thin sweaters feel warmer than one thick sweater.
(c) Utensils are made of metal, while their handles are made of plastic.
Q.1 Radiation
(A) does not require a material medium
(B) is the process of the transfer of heat in liquids
(C) is the process of the transfer of heat in which heat travels in one direction
(D) occurs in solids
Q.2 Which of the following statements is correct?
(A) Metals are bad conductors.
(B) Some metals conduct heat better than others.
(C) Heat can be conducted from one metal to another even if they are not in contact with each other.
(D) When two metal rods are placed in contact with each other. heat can flow from one to the other even if they are at the same temperature.
Q.3 The capillary tube of a clinical thermometer has a kink
(A) to increase the expansion of mercury
(B) so that the level of mercury does not fall as soon as the thermometer is taken out of the mouth
(C) to use less mercury
(D) to help us see it better
Q.4 A polished. silvery surface is a
(A) good absorber and good reflector of heat
(B) good absorber and bad radiator of heat
(C) poor absorber and good reflector of heat
(D) poor reflector and good radiator of heat
Q.5 A black body with a rough surface is a good
(A) reflector and poor absorber of heat
(B) good absorber and good radiator of heat
(C) absorber and poor radiator of heat
(D) reflector and poor radiator of heat
Q.6 Sweating causes cooling by
(A) conduction (B) radiation
(C) convection (D) evaporation
Q.7 Air conditioners are placed high up the walls so that-
(A) they create less noise
(B) cold air from them comes down and cools the room while hot air rises up
(C) They are out of the way
(D) all of the above
Q.8 It is easier to drink hot tea from a porcelain mug than from a steel tumbler as
(A) the porcelain mug has a handle
(B) porcelain is a heat insulator
(C) the tea will cool faster in the steel tumbler
(D) all of the above
Q.9 The heat energy emitted by sun reaches the earth through-
(A) conduction (B) convection
(C) radiation (D) None of these
Q.10 Ice blocks are covered with sawdust because-
(A) sawdust lowers the temperature of the ice
(B) the sawdust, together with the air trapped within it, acts as heat insulator and prevents the heat outside from getting to the ice and melting it
(C) the sawdust absorbs excess water from the ice
(D) None of these
Q.11 Land and sea breezes are based on-
(A) the phenomenon of conduction of heat
(B) the phenomenon of convection of heat
(C) the phenomenon of absorption and radiation of heat
(D) all of the above
Q.12 1 calorie equals to-
(A) 4.2 J (B) 0.42 J
(C) 420 J (D) 4200 J
Q.13 Fahrenheit scale divides two fixed point into-
(A) 180 parts (B) 212 parts
(C) 100 parts (D) 32 parts
Q.14 The normal temperature of human body is-
(A) 37°C (B) 38°C
(C) 35°C (D) 98.4°C
Q.15 Convert 293 K into Celsius scale-
(A) 566°C (B) 293°C
(C) 20°C (D) 496°C
Q.16 When in thermal contact, the quantity of heat lost by the hotter body is -------- the amount of heat gained by the colder body.
(A) equal to (B) greater than
(C) less than (D) cannot say
Q.17 Conduction cannot takes place in
(A) copper (B) iron
(C) aluminium (D) vacuum
Q.18 The snow on the mountains does not melt all the at once when it is heated by the sun because it-
(A) becomes very hard
(B) reflects most of the heat from the sun
(C) has a low specific heat capacity
(D) has a high latent heat of fusion
ANSWER KEY
1. A 2. B 3. B 4. C 5. B
6. D 7. B 8. B 9. C 10. B
11. B 12. A 13. A 14. A 15. C
16. A 17. D 18. D
1. When we touch a steel rod and a paper simultaneously, we feel that the rod is colder because:
(A) Iron being a good conductor conducts more heat from our body
(B) paper being a good conductor conducts more heat from our body
(C) more heat flows from the iron to our body
(D) more heat flows from the paper to our body
2. When you heat the water in a pot, it boils. What do you infer from above observation?
(A) Heat is a form of energy
(B) Water can boil itself
(C) Water develops heat on it own
(D) None of the above
3. The lower fixed point on the Celsius scale is:
(A) melting point of ice
(B) boiling point of water
(C) melting point of mercury
(D) mean of melting point and boiling point of water
4. In the Celsius scale, the upper fixed point is:
(A) melting point of ice
(B) boiling point of water
(C) boiling point of mercury
(D) mean of melting point and boiling point of water
5. When an object is heated, it generally:
(A) expands
(B) contracts
(C) remains same
(D) may expand or contract
6. The capacity to do work is called:
(A) force
(B) movement
(C) energy
(D) momentum
7. The measure of degree of hotness or coldness of a body is called:
(A) Heat energy
(B) Celsius
(C) Kelvin
(D) Temperature
8. In which of the following, chemical energy is converted into heat energy?
(A) Heater
(B) Refrigerator
(C) Candle
(D) Motor
9. Which of the following expands most of heating?
(A) Solids
(B) Liquids
(C) Sand ages
(D) Gases
10. In electric heater:
(A) chemical energy is converted to electrical energy
(B) electrical energy is converted to chemical energy.
(C) heat energy is converted to electrical energy
(D) electrical energy is converted to heat energy
11. In steam engine:
(A) mechanical energy is converted to heat energy
(B) heat energy is converted to mechanical energy
(C) heat energy is converted to electrical energy
(D) heat energy is converted to chemical energy
12. Mercury is widely used in clinical thermometers because:
(A) mercury is cheap
(B) mercury is clearly visible
(C) is fashionable to use mercury
(D) mercury has a constant coeffi- cient of expansion
13. The CGS unit for energy is:
(A) dyne (B) erg
(C) centimeter (D) joule
14. According to the law of conservation of energy:
(A) energy exists in only one form
(B) energy can be created but no destroyed, and it can be trans- formed from one form to another
(C) energy exists in many forms but it cannot be transformed
(D) energy can neither be produced nor be destroyed and it can be transformed from one form to an-other form
15. Chemical reactions produce heat energy if:
(A) energy absorbed by reactants is more than the energy released by products
(B) energy absorbed by reactants is less than the energy released by products
(C) energy absorbed by reactants is equal to the energy released by products
(D) all of these
16. When the temperature of a substance is increased:
(A) kinetic energy of the molecules of the substance increases
(B) kinetic energy of the molecules of the substance decreases
(C) the amplitude of vibrations of molecules of the substance about their mean position decreases
(D) it does not show any change
17. Kinetic energy of a gas molecule depends on:
(A) Pressure (B) volume
(C) temperature (D) all the above
18. Which of the following is not a scale of temperature?
(A) Kelvin scale
(B) Celsius scale
(C) Fahrenheit scale
(D) Richter scale
19. The difference between lower fixed point d upper fixed point is divided into …… parts on a Celsius scale.
(A) 100 (B) 273
(C) 180 (D) 50
20. On a Fahrenheit scale:
(A) boiling point of water is 212°F
(B) the temperature will be equal to that on the Celsius scale at- 40°C
(C) the difference between the upper fixed point and the lower fixed point is divided into 180 equal parts
(D) all the above
21. The range of a clinical thermometer 1s:
(A) 0-1000C (B) 32-214°F
(C) 0-2730C (D) 35-420C
22. Human body temperature is nor-mally:
(A) 32°F (B) 212°F
(C) 100.40F (D) 98.6°F
23. We cannot use mercury thermo- meter at low temperatures because:
(A) glass might break down at low temperature
(B) heat does not now from the body whose measurement we are taking with the thermometer
(C) at low temperatures mercury becomes transparent and it becomes difficult to take the readings
(D) mercury freezes at low temperatures.
24. Iron glows in red colour when it is heated to very high temperature because:
(A) heat we supply assumes red colour at high temperature
(B) mechanical energy is being con-verted into heat energy
(C) all metals glow in red colour when heated
(D) heat energy is being converted into light energy
25. According to law of conservation of energy the total energy of the universe is:
(A) increasing
(B) decreasing
(C) remains constant
(D) above law does not say anything about the universe
26. People boil water as a safe measure
to drink because:
(A) boiled water is tasty
(B) boiled water is more powerful
(C) heating water is fashionable
(D) excessive heat kills the microorganisms present in the water
27. Iron, hydrogen peroxide and carbon dioxide. When same amount heat is supplied to these three substances, which expands most?
(A) Hydrogen peroxide
(B) Carbon dioxide
(C) Iron
(D) All of these
28. Expansion of a substance on heating depends on:
(A) nature of the substance
(B) rise in temperature
(C) both A and B
(D) colour of the substance
29. On a cold day, it is hard to open the lid of a tight container. But when you gently heat the neck you can easily open the lid. Why?
(A) On heating glass expands and lid contracts
(B) Lid expands more than the neck and thus slides easily
(C) Neck becomes slippery on heating
(D) Lids of the bottles cannot bear the heat
30. Cement floors are laid with the glasses between the rectangular floor tiles. The reason is:
(A) to give them a beautiful geometrical design
(B) glasses hold the floor tiles strongly
(C) not to let the floor tiles crack on heating in the summer
(D) there is no specific reason
31. Gaps are left between railway tracks because:
(A) gaps give the space to the racks to expand in summer heat
(B) gaps hold the tracks firmly
(C) to produce gentle rhythmic sound when the train moves on the track
(D) it is customary to leave the gaps
32. Electric wires sag in summer and become taut in the winter. Reason?
(A) They perform simple harmonic oscillations according to changes in the seasons
(B) They expand due to summer heat and contract due to winter cold
(C) They expand due to winter cold
and contract due to summer heat
(D) There is no specific reason
33. Mercury is the ideal liquid to use in a thermometer because:
(A) it does not stick to glass and is visible
(B) expands a lot on heating
(C) it has a high boiling tempera- ture
(D) all the above
34. At low temperatures ….. type of thermo meter is used.
(A) mercury thermometer
(B) water thermometer
(C) alcohol thermometer
(D) thermometers cannot be used
35. A dog pants with its tongue sticking out on a hot day. The reason is:
(A) it is its habit
(B) producing more saliva cools down the body temperature
(C) saliva vapourises, cooling the tongue
(D) it is a genetic disease found in few animals like dogs
36. Process of change of state from solid to liquid is called:
(A) melting (B) freezing
(C) boiling (D) condensation
37. Process of change of state from liquid to solid is called:
(A) melting (E) freezing
(C) boiling (D) condensation
38. Process of change of state from gaseous state to liquid state is called:
(A) freezing (B) sublimation
(C) boiling (D) condensation
39. Then a substance changes its state type of from lid to liquid:
(A) the temperature remains constant
(B) there is a constant rise in temperature
(C) there is a constant drop in tem-perature
(D) the temperature fluctuates
40. Sublimation is:
(A) increasing the energy of a substance until the chemical reaction starts
(B) process of change of state from gas to liquid
(C) process of change of state from solid to gas
(D) process of preparing water vapor even below the boiling point by subjecting it to extra pressure
41. The rise in temperature of a substance depends on:
(A) the quantity of heat supplied
(B) the mass of the substance
(C) the nature of the substance
(D) all the above
42. The temperature of the substance remains constant when it is melting and boiling though some quantity of this energy?
(A) It is dissipated as sound energy
(B) It is consumed to increase the energy of the molecules
(C) It is used to change the state of the substance
(D) It is still an unsolved problem in science
43. 1 cal = ________.
(A) 10 joules (B) 4.18 joules
(C) 4.18 dynes (D) none of these
44. 1 calorie =
(A) amount of heat energy required to transform 1 kg of ice into water at 0C
(B) amount of heat energy required to raise the temperature of 1 kg of water through 1°C
(C) amount of heat required to raise the temperature of l g of water through 1°C
(D) quantity of work done at atmospheric pressure
45. The SI unit of heat IS:
(A) calorie (B) joule
(C) dyne (D) Newton
46. When in thermal contact, the quantity of heat lost by the hotter body is …… the amount of heat gained by the colder body.
(A) equal to (B) greater than
(C) less than (D) cannot say
47. Which of the following would expand more on heating?
(A) Carbon dioxide
(B) Hydrogen peroxide
(C) Calcium hydroxide
(D) Calcium carbonate
48. When two bodies at different temperatures are placed in thermal contact with each other, heat flows from the body at higher temperature to the body at lower temperature until they both acquire the same temperature. Assuming that there is no loss of heat to the surroundings, the heat:
(A) gained by the hotter body will be equal to the heat lost by the colder body
(B) the heat gained by the hotter body will be less than the heat lost by the colder body
(C) the heat gained by the hotter body will be greater than the heat lost by the colder body
(D) the heat lost by the hotter body will be equal to the heat gained by the colder body.
49. Which of the following is a good conductor of heat?
(A) Plastic (B) Water
(C) Glass (D) Copper
50. In solid substances, heat is trans- ferred by:
(A) conduction (B) convection
(C) radiation (D) none of these
51. Which of the following is a bad con- ductor of heat?
(A) Wood (B) Aluminium
(C) Iron (D) Bronze
52. When two objects are in thermal contact, the heat is transferred by:
(A) conduction (B) convection
(C) radiation (D) none of these
53. The handles of cooking vessels are covered with plastic or wood because:
(A) they are beautiful
(B) it is customary
(C) they are good conductors of heat
(D) they are bad conductors of heat
54. The instrument used to detect the radiation of heat is:
(A) thermo scope (B) barometer
(C) stethoscope (D) cinemascope
55. We receive heat energy from the sun through …….. mode of transmission:
(A) conduction (B) convection
(C) radiation (D)yet to be found
56. Which of the following are good conductors of heat?
(A) Metals (B) Glass
(C) Water (D) Wood
57. Convection of heat takes place in:
(A) metals only (B) liquids only
(C) gases only (D)liquids & gases
58. Which one of the following is a good conductor of heat?
(A) Bromine (B) Alcohol
(C) Mercury (D) Water
59. Which of the following statement is true?
(A) Metal are bad conductors
(B) In gases, heat is transferred by conduction
(C) In conduction, molecules move from hotter to colder parts
(D) Metals conduct heat better than The gases
60. Conduction is:
(A) the process of transfer of heat which does not require any material medium
(B) the process of transferring heat from molecule to molecule, with out any movement of molecules
(C) the process in which molecules conduct heat by moving ‘from hotter regions to colder regions
(D) the process in which heat energy moves through the medium of protons
61. Conduction is possible:
(A) when the bodies are apart from each other
(B) when the bodies have same temperature and in thermal contact
(C) when they have different temperatures maintaining distance between them
(D) bodies should be in contact and should have different temperatures
62. A polished, silvery surface is a:
(A) good absorber and good reflector of heat
(B) good absorber and bad radiator of heat
(C) poor absorber and good reflector of heat
(D) poor reflector and good radiator of heat
63. In conduction, heat flows from:
(A) hotter to hotter region
(B) colder to hotter region
(C) hotter to colder region
(D) colder to colder region
64. More energetic molecules of a body transfer some of their energy to other molecules, without any change
(A) convection (B) convection
(C) radiation (D) none of these
65. Substances which allow heat to pass
through them are called:
(A) conductors (B) insulators
(C) moderators (D) none of these
66. The best conductor of heat is:
(A) silver (B) iron
(C) aluminum (D) copper
67. A coin is dipped in the molten wax in a glass tube. When we heat the upper part of the glass tube, the wax around the coin will not melt because:
(A) wax has a very high melting point
(B) wax is a good conductor of heat
(C) glass is a good conductor of heat
(D) wax and glass are bad conduct- ors
68. The liquid which is a good conductor of heat is:
(A) mercury (B) water
(C) hydrogen (D) kerosene
69. Cups are not made of metals, The reason is:
(A) metals are good conductors
(B) metals are bad conductors
(C) metals are expensive
(D) none of the above
70. When you pour hot tea suddenly on to a glass tumbler, it cracks. Because:
(A) glass cannot resist heat
(B) glass expands unevenly around the place of contact with the tea
(C) tea has chemicals which react with glass tumblers.
(D) the reason is yet to be discovered by the scientists
71. Thermo flasks and doors of refrigerator have two layered structure with an insulator in between so as to:
(A) allow more heat to pass through it
(B) not to let heat flow through it
(C) to make it more stronger
(D) beautify it
72. When you heat a bowl of water, we can observe that water starts circulating. This demonstrates:
(A) conduction (B) convection
(C) radiation (D) none
73. It is warmer to have two thin blankets than to have a single thick blanket because:
(A) thick blankets cannot give more warmth
(B) two blankets allow more heat to
pass through them
(C) air between the two blankets is a good conductor of heat
(D) air between the thin blankets does not allow heat to pass through it since it is a bad conductor
74. Warmer air is:
(A) lighter than cold air
(B) heavier than cold air
(C) both have equal weights
(D) we cannot say
75. Rooms are fitted with ventilators to let the air move around‘ The phenomenon involved is:
(A) conduction (B) convection
(C) radiation (D) condensation
76. Firemen crawl when entering a burning building because:
(A) it is easier to crawl
(B) smoke rises high in the air
(C) it helps to move faster
(D) to resist more heat
77. Birds glide effortlessly in the air with the help of:
(A) conduction of heat in the air
(B) radiation of light through the atmosphere
(C) convection currents of air
(D) more sugar
78. The phenomenon involved in sea breeze and land breeze is:
(A) convection (B) conduction
(C) radiation (D) none
79. The heat energy emitted by sun reaches the earth through:
(A) conduction (B) convection
(C) radiation (D) none
80. Electric heater converts:
(A) heat energy into electrical energy
(B) electrical energy into heat energy
(C) chemical energy into heat energy
(D) heat energy into Chemical energy
81. White objects:
(A) absorb more light
(B) absorb less light
(C) radiate light
(D) none of the above
82. Black bodies are:
(A) good absorbers and bad radiators
(B) good absorbers and good radiators
(C) bad absorbers and good radiators
(D) bad absorbers and bad radiators
83. It is better to wear white clothes in summer because:
(A) they are cheap
(B) the absorb more light
(C) they absorb less light
(D) they are good looking
84. Reflecting solar films are used on the top of the cars to:
(A) produce electricity
(B) to absorb more light
(C) to prevent heating by radiation
(D) to make to strong
85. A thermos: flask:
(A) keeps hot liquids hot and cold liquids hot
(B) keeps cold liquids hot and hot liquids cold
(C) keeps both COM and hot iquids
cold
(D) keeps cold liquids cold and hot liquids hot
86. In solar cooker:
(A) electricity is used to ‘cook food
(B) Chemical energy is converted to electrical energy
(C) radiation from the sum is used to cook food
(D) heat energy is converted light energy
87. Conduction cannot take place in:
(A) copper (B) iron
(C) aluminum (D) vacuum
88. Radiation depends on:
(A) the temperature, of the substance
(B) the colour of the substance
(C) both the above
(D) none of these
1. A 2. A 3. A 4. B 5. A 6. C 7. D
8. C 9. D 10. D 11. B 12. D 13. B 14. D
15. B 16. A 17. C 18. D 19. A 20. D 21. D
22. D 23. D 24. D 25. C 26. D 27. B 28. C
29. B 30. C 31. A 32. B 33. D 34. C 35. C
36. A 37. B 38. D 39. A 40. C 41. C 42. C
43. B 44. C 45. B 46. A 47. A 48. D 49. D
50. A 51. A 52. A 53. D 54. A,C 55. C 56. A
57. D 58. C 59. D 60. C 61. D 62. C 63. C
64. A 65. A 66. A 67. D 68. A 69. A 70. B
71. B 72. B 73. D 74. A 75. B 76. C 77. C
78. A 79. C 80. B 81. B 82. B 83. C 84. C
85. D 86. C 87. D 88. C