Solved Examples
EXAMPLE 27.1
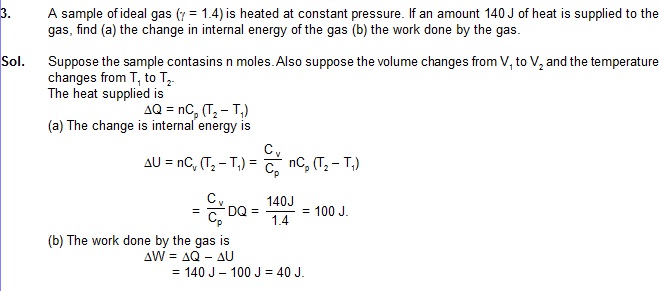
0.32 g of oxygen is kept a rigid container and is heated. Find the amount of heat needed to raise the temperature from
250 C to 350 C. The molar heat capacity of oxygen at constant volume is 20 J/mol–K.
Sol.
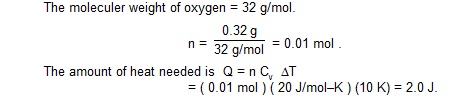
EXAMPLE 27.2
A tank of volume 0.2 m 3 contains helium gas at a temperature of 300 K and pressure 1.0 × 10 5 N/m 2 . Find the amount
of heat required to raise the temperature to 400 K. The molar heat capacity of helium at constant volume is 3.0 cal/mol–K.
Neglect any expansion in the volume of the tank.
Sol.
The amount of the gas in moles is
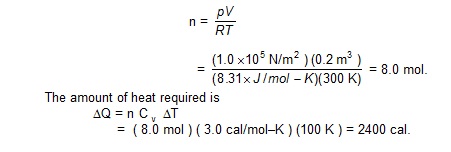

EXAMPLE 27.4
Dry air at 150 C and 10 atm is suddenly released at atmosphere pressure. Find the final temperature of the air [ CP / Cv = 1.4 ].
Sol.
As the air is suddenly released, it does not get time to exchange heat with the surrounding. Thus the process is adiabatic. Assuming the process to be reversible, 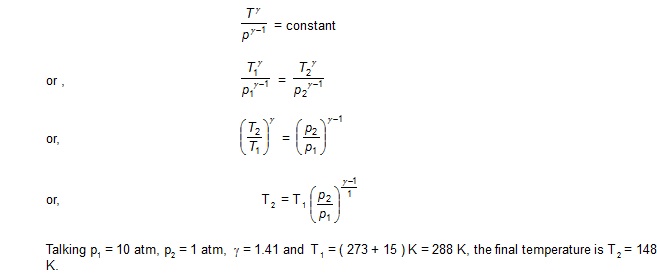
EXAMPLE 27.5
Calculate the internal energy of 1 g of oxygen at STP.
Sol.
Oxygen is a diatomic gas. The average energy per molecules is, therefore,5/2 kT and the average energy per mole is 5/2 RT.
As the molecular weight of oxygen is 32 g/mol, 1 g of oxygen has
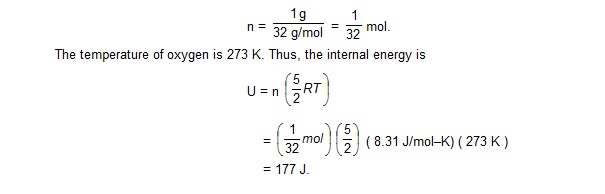
Questions for Short answer
1. Does a gas have just two specific heat capacities or more than two? Is the number of specific heat capacities of a gas countable?
2. Can we define specific heat capacity at constant temperature?
3. Can we define specific heat capacity for an adiabatic process?
4. Does a solid also have two kinds of molar heat capacities Cp and Cv ? If yes, do we have Cp > Cv ? Cp – Cv = R ?
5. In a real gas the internal energy depends on temperature and also on volume. The energy increases when the gas expands isothermally.
Looking into the derivation of Cp – Cv = R, find whether Cp – Cv will be more than R, less than R orequal to R for a real gas.
6. Can a process on an ideal gas be both adiabatic and isothermal?
7. Show that the slope of p–V diagram is greater for an adiabatic process as compared to an isothermal process.
8. Is a slow process always isothermal? Is a quick process always adiabatic?
9. Can two states of an ideal gas be connected by an isothermal process as well as an adiabatic process?
10. The ratio Cp/Cv for a gas is 1.29. What is the degree of freedom of the molecules of this gas?
Objective - I
1. Work done by a sample of an ideal gas in a process A is double the work done in another process B.
The temperature rises through the same amount in the two processes. If CA and CB be the molar heat
capacities for the two processes.
(A) CA = CB (B) CA < CB (C*) CA > CB (D) CA and CB cannot be defined
2. For a solid with a small expansion coefficient,
(A) Cp - Cv = R (B) Cp - Cv = R (C*) Cp is slightly greater than Cv
(D) Cp is slightly less than Cv
3. The value of Cp - Cv is 1.00 R for a gas sample in state A and is 1.08 R in state B. Let pA, pB denote the
pressure and TA and TB donote the temperature of the states A and B respectively. Most likely
(A) pA < pB and TA>TB (B) pA > pB and TAB (C) pA = pB and TAB (D) pA > pB and TA=TB
4. Let Cv and Cp denote the molar heat capacities of an ideal gas at constant volume and constant pressure respectively.
Which of the following is a universal constant ?
(A) Cp/Cv (B) CpCv (C) Cp - Cv (D) Cp + Cv
5. 70 calories of heat is required to raise the temperautre of 2 mole of an ideal gas at constant pressure from 30oC to 35oC.
The amount of heat required to raise the temperature of the same gas through the same range at constant volume is -
(A) 30 Calories (B) 50 Calories (C) 70 Calories (D) 90 Calories
6. Fig shows a process on a gas in which pressure and volume both change. The molar heat capacity for this process is c.
(A) C = 0 (B) C = Cv (C) C > Cv (D) C < Cv
7. The molar heat capacity for the process shown in fig. is
(A) C = Cp (B) C = Cv (C) C > Cv (D) C = 0
8. In an isothermal process on an ideal gas, the pressure increases by 0.5%. The volume decreases by about
(A) 0.25% (B) 0.5% (C) 0.7% (D) 1%
9. In an adiabatic process on a gas with g = 1.4, the pressure is increased by 0.5%. The volume decreases by about
(A) 0.36% (B) 0.5% (C) 0.7& (D) 1%
10. Two sample A and B are initially kept in the same state. The sample A is expanded through an adiabatic process and the
sample A is expanded through an adiabatic process and the sample B through an isothermal process.
The final volumes in A and B are pA and pB respectively.
(A) pA > pB (B) pA = pB (C*) pA < pB
(D) The relation between pA and pB cannot be deduced.
11. Let Ta and Tb be the final temperature of the samples A and B respectively in the previous question then :
(A) Ta < Tb (B) Ta = Tb (C*) Ta > Tb
(D) The relation between Ta and Tb cannot be deduced.

13. The molar heat capacity of oxygen gas at STP is nearly 2.5 R. As the temperature is increased, it gradually
increases and approaches 3.5 R. The most appropriate reason for this behaviour is that at high temperatures
(A) oxygen does not behave as an ideal gas (B) oxygen molecules dissociate in atoms
(C) the molecules collides more frequently (D*) molecular vibration gradually become effective
Objective - II
1. A gas kept in a container of finite conductivity is suddenly compressed. The process
(A) must be very nearly adiabatic (B) must be very nearly isothermal
(C) may be very nearly adiabatic (D) may be very nearly isothermal
Sol. If container is a very good conductor (D) is correct.
If container is very bad conductor (C) is correct.
2. Let Q and W denote the amount of the heat given to an ideal gas and the work done by it in an isothermal process.
(A) Q = 0 (B) W = 0 (C) Q ¹ W (D) Q = W
3. Let Q and W denote the amount of the heat given to an ideal gas and the work done by it in an adiabatic process.
(A) Q = 0 (B) W = 0 (C) Q ¹ W (D) Q = W
4. Consider the processes A and B shown in fig. It is possible that
(A) both the processes are isothermal (B) both the processes are adiabatic
(C) A is isothermal and B is adiabatic (D) A is adiabatic and B is isothermal
5. Three identical adiabatic containers A, B and C contain helium, neon and oxygen respectively at equal pressure. The gases are pushed to half their original volumes.
(A) The final temperature in the three containers will be the same.
(B) The final pressures in the three containers will be the same.
(C) The pressure of helium and neon will be the same but that of oxygen will be different.
(D) The temperature of helium and neon will be the same but that of oxygen will be different
6. A rigid container of neligible heat capacity contains one mole of an ideal gas. The temperature of the gas increases by 10 C if 3.0 cal of heat is added to it. The gas may be
[4 min.]
(A) helium (B) argon (C) oxygen (D) carbon dioxide
7. Four cylinders contain equal number of moles of argon, hydrogen, nitrogen and carbon dioxide at the same temperature. The energy is minimum in
(A) argon (B) hydrogen (C) nitrogen (D) carbon dioxide
Worked Out Examples
1. Calculate the value of mechanical equialent of heat from the following data. Specific heat capacity of air at
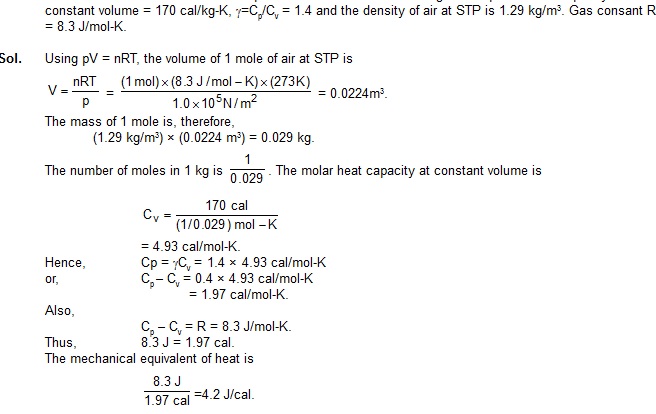
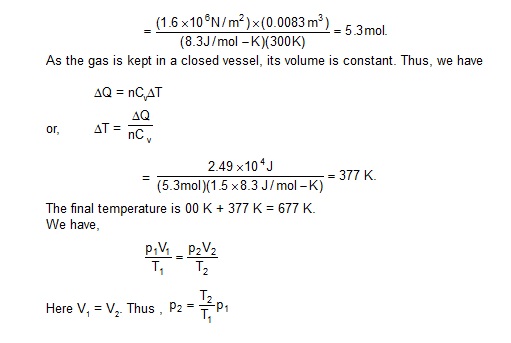
2. An ideal gas has a molar heat capacity at constant pressure Cp = 2.5 R. The gas is kept in a closed vessel of volume 0.0083 m3,
at a temperature of 300 K and a pressure of 1.6 × 106 N/m2. An amount 2.49 × 104 J of heat energy is supplied to the gas.
Calculate thefinal temperature and pressure of the gas.
Sol. We have
Cv = Cp – R = 2.5 R – R = 1.5 R.
The amount of the gas (in moles) is n = pV/RT
As the gas is kept in a closed vessel, its volume is constant. Thus, we have
4. An experiment is performed to measure the molar heat capacity of a gas at constant pressure using Regnault’s method.
The gas is initially contained in a cubical reservoir of size 40cm × 40 cm × 40 cm at 600 kPa at 27ºC. A part of the gas is
brought out, heated to 100ºC and is passed through a calorimeter at constant pressure. The water equivalent of the calorimeter
and its contents is 100g. The temperature of the calorimeter and its contents increases from 20ºC to 30ºC during the experiment
and the pressure in the reservoir decreases to 525 kPa. Specific heat capacity of water = 4200 J/kg–K. Calculate the molar heat
capacity Cp from these data.
Sol. We have pV = nRT or, n = . The amount of the gas in the reservoir is n1 = before the gas is taken out and after the gas is
taken out. The amount taken out is
Dn = n1 – n2 = (p1 – p2)
=
= 1.925 mol.
The gas is heated to 100ºC and cools down as it passes through the calorimeter. The average final temperature of the gas is =25ºC.
Thus, the average decrease in temperature of the gas is
DT = (100ºC – 25ºC) = 75ºC
or, DT = 75 K.
The heat lost by the gas is
DQ = Dn Cp DT.
The heat gained by the calorimeter and its contents is
(100 g) (4200 J/kg–K) (30 – 20)ºC = 4200 J.
Thus, Dn Cp DT = 4200 J
or,
= 29 J/mol-K.
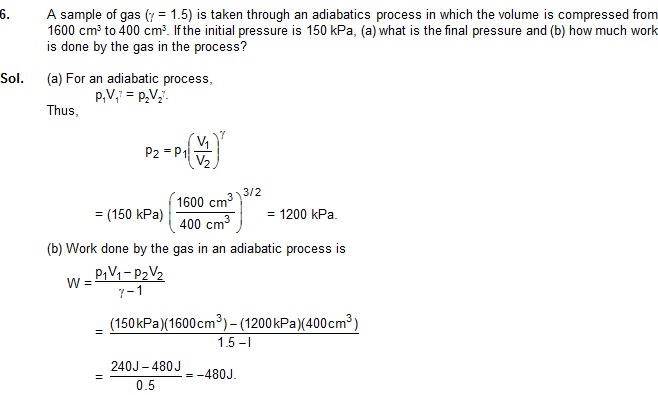
5. A quantity of air is kept in a container having walls which are slightly conducting. The initial temperature and volume are 27ºC
(equal to the temperature of the surrounding) and 800cm3 respectively. Find the rise in the

Rise in temperature = T2 – T1 = 222 K.
(b) When the gas is compressed in a long time, the process is isothermal. Thus, the temperature remains equal to the temperature
of the surrounding that is 27ºC. The rise in temperature = 0.
7. Two moles of helium gas (g = 5/3) are initially at 27ºC and occupy a volume of 20 litres. The gas is first expanded at constant
pressure until the volume is doubled. Then it undergoes an adiabatic change unitil the temperature returns to its initial value.
(a) Sketch the process in a p-V diagram.
(b) What is the final volume and pressure of the gas?
(c) What is the work done by the gas?
Sol. (a) The process is shown in figure. During the part ab, the pressure is constant. We have
paVa /Ta= pcVc/Tb
or, Tb = Vb /Va
During the part bc, the gas is adiabatically returned to the temperature Ta. The point a and the point c are on the same iotherm.
Thus, we draw an adiabatic curve from b and an isotherm from a and look for the point of intersection c. That is the final state.
(b) From the isotherm ac,
paVa /Ta= pcVc/Tb ...... (i)
and from the adiabatic curve bc
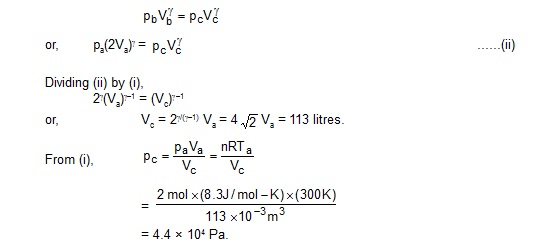
(c) Work done by the gas in the part ab
= pa (Vb – Va)
= pbVb – paVa
= nRT2 – nRT1
= 2 mol × (8.3 J/mol-K) × (600 K – 300 K)
= 4980 J.
The work done in the adiabatic part bc
![]()
The net work done by the gas
= 4980J + 7470 J = 12450 J.
8. An ideal gas enclosed in a vertical cylindrical container supports a freely moving piston of mass M. The piston and the
cylinder have equal cross-sectional area A. When the piston is in equilibrium, the volume of the gas is V0 and its
pressure is p0. The piston is slightly displaced from the equilibrium position and released. Assuming that the system
is completely isolated from its surrounding, show that the piston executes simple harmonic motion and find the
frequency of oscillations.
Sol. Suppose the piston is displaced through a distance x above the equilibrium position. The volume of the gas
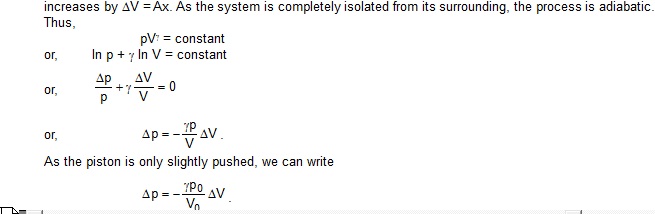 .
.
The resultant force acting on the piston in this position is
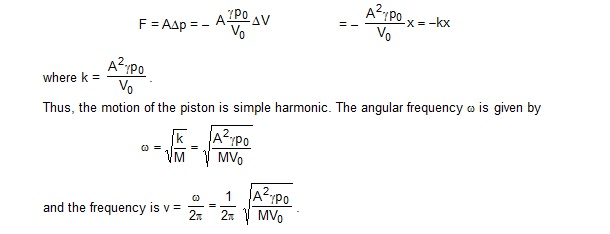
.
9. Two vessels of volumes V1 and V2 contain the same ideal gas. The pressures in the vessels are p1 and p2 and the
temperatures are T1 and T2 respectively. The two vessels are now connected to each other through a narrow tube.
Assuming that no heat is exchanged between the surrounding and the vessels, find the common pressure and
temperatures attained after the connection.
Sol.
The amount of the gas in vessel 1 is n1 = p1V1/RT
and that in vessel 2 is n2.= p2V2/RT2
If p' and T’ be the common pressure and temperature after the connection is made, the amounts are
n1= pV1/RT = and n2 = pV2/RT
We have n1 + n2 = n1 + n2
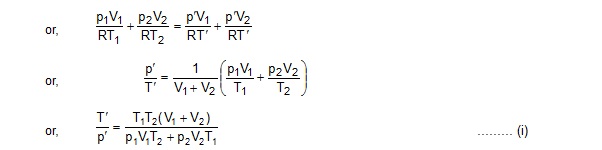
As the vessels have fixed volume, no work is done by the gas plus the vessels system. Also, no heat is exchanged
with the surrounding. Thus, the internal energy of the total system remains constant.
The internal energy of an ideal gas is
U = nCvT
= Cv pV/R.
The internal energy of the gases before the connection

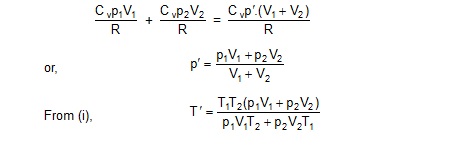
![]()
Sol. Let,
Cv’ = molar heat capacity of the first gas,
= molar heat capacityof the second gas,
Cv = molar heat capacity of the mixture
and similar symbols for other quantities. Then,
gamma Cp/Cv= 1.67
and Cp = Cv + R
This gives Cv = 3/2R and Cp = 5/2R.
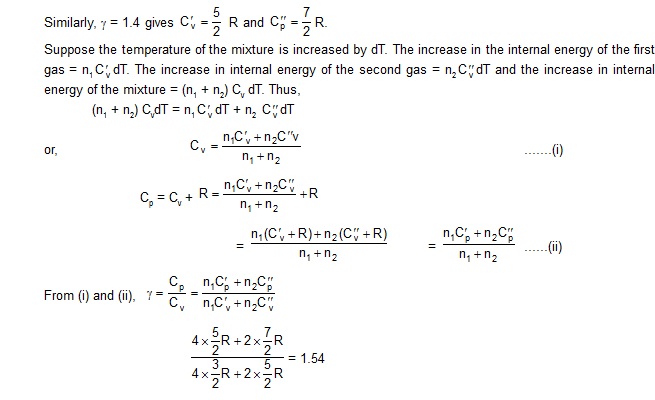

Sol. For a diatomic gas, Cv = 5/2 R and Cp = 7/2 R. The work done in an isobaric process is
W = p(V2 – V1)
= nRT2 – nRT1
or T2 – T1 = .W/nR
The heat given in an isobaric process is
Q = nCp (T2 – T1)
= nCp W/nR = 7/2W = 7/2 x 200J = 700 J.
12. Calculate the ratio Cp / Cv of oxygen from the following data. Speed of sound in oxygen at 0ºC= 315 m/s,
molecular weigth of oxygen = 32 g/mol and the gas constant R = 8.3 J/mol-K.
Sol. The speed of sound in a gas is given by
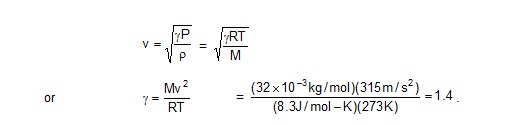
Exercise
1. A vessel containing one mole of a monatomic ideal gas (molecular weight = 20 g/mol) is moving on a floor at a speed of 50m/s.
The vessel is stopped suddenly. Assuming that the mechanical energy lost has gone into the internal energy of the gas,
find the rise in its temperature.
[Ans. 2.0K]
2. 5 g of a gas is contained in a rigid container and is heated from 15ºC to 25ºC. Specific heat capacity of the gas at constant volume
is 0.172 cal/g–ºC and the mechanical equivalent of heat is 4.2 J/cal. Calculate the change in the internal energy of the gas.
[Ans. 36J]
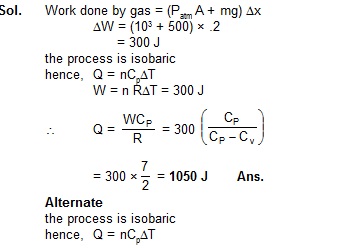
3. Figure shows a cylindrical container with vertical axis containing oxygen (g = 1.4) and closed by a 50 kg frictionless piston.
The area of cross-section is 100 cm2, atmospheric pressure is 100 kPa and g is 10 m/s2. The cylinder is slowly heated for
some time. Find the amount of heat supplied to the gas if the piston moves out through a distance of 20 cm [2]
![]()
4. The specific heat capacities of hydrogen at constant volume and at constant pressure are 2.4 cal/g–ºC and 3.4 cal/g–ºC respectively.
The molecular weight of hydrogen is 2 g/mol and the gas constant R = 8.3 × 107 erg/mol–ºC. Calculate the value of J.
[Ans. 4.15 × 107 erg/cal]
5. The ratio of the molar heat capacities of an ideal gas is Cp / Cv = 7/6. Calculate the change in internal energy of 1.0 mole of the
gas when its temperature is raised by 50 K
(a) keeping the pressure constant,
(b) keeping the volume constant and (adiabatically).
[Ans. 2490 J in all cases]
6. A sample of air weighing 1.18g occupies 1.0 × 103 cm3 when kept at 300 K and 1.0 × 105 Pa. When 2.0 cal of heat is added to
it at constant volume, its temperature increases by 1ºC. Calculate the amount of heat needed to increases the temperature of air
by 1ºC at constant pressure if the mechanical equivalent of heat is 4.2 × 107 erg/cal. Assume that air behaves as an ideal gas.
[Ans. 2.8 cal]
7. An ideal gas expands from 100 cm3 to 200 cm3 at a constant pressure of 2.0 × 105 Pa when 50 J of heat is supplied to it. Calculate
(a) the change in internal energy of the gas,
(b) the number of moles in the gas if the initial temperature is 300 K,
(c) the molar heat capacity Cp at constant pressure and
(d) the molar heat capacity Cv at constant volume.
[Ans. (a) 30 J, (b) 0.008, (c) 20.8 J/mol–K, (d) 12.5 J/mol–K]
8. An amount Q of heat is added to a monotomic ideal gas in a process in which the gas perform a work Q/2 on its surrounding.
Find the molar heat capacity for the process.
[Ans. 3 R]
9. An ideal gas is taken through a process in which the pressure and the volume are changed according to the equation p = kV.
Show that the molar heat capacity of the gas for the process is given by C = Cv + R/2 .
10. An ideal gas (Cp/Cv = gamma) is taken through a process in which the pressure and the volume vary as p = aVb.
Find the value of b for which the specific heat capacity in the process is zero.
[Ans. –gamma]
11. Two ideal gases have the same value of Cp/Cv = gamma. What will be the value of this ratio for a mixture of the two gases in the ratio 1 : 2?
[Ans. g]
12. A mixture contains 1 mole of helium (Cp = 2.5R, Cv = 1.5 R) and 1 mole of hydrogen (Cp = 3.5 R, Cv = 2.5 R) and 1 mole of
hydrogen (Cp = 3.5 R, Cv = 2.5 R). Calculate the value of Cp, Cv and gamma for the mixture.
[Ans. 3R, 2R, 1.5]
13. Half mole of an ideal gas (gamma = 5/3) is taken through the cycle abcda as shown in figure. Take R = 25/3 J/mol–K
(a) Find the temperature of the gas in the states a, b, c and d.
(b) Find the amount of heat supplied in the processes ab and bc.
(c) Find the amount of heat liberated in the processes cd and da.
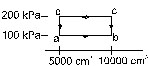
[Ans. (a) 120 K, 240 K, 480 K, 240 K, (b) 1250 J, 1500 J, (c) 2500 J, 750 J]
14. An ideal gas (gamma = 1.67) is taken through the process abc shown in figure. The temperature at the point a is 300 K. Calculate
(a) the temperatures at b and c,
(b) the work done in the process,
(c) the amount of heat supplied in the path ab and in the path bc and
(d) the change in the internal energy of the gas in the process.
[Ans. (a) 600 K, 900 K, (b) 10 J, (c) 14.9 J, 24.9 J, (b) 29.8]
1
5. In Joly’s differential steam calorimeter, 3g of an ideal gas is contained in a rigid closed sphere at 20ºC.
The sphere is heated by steam at 100ºC and it is found that an extra 0.095 g of steam has condensed into
water as the temperature of the gas becomes constant. Calculate the specific heat capacity of the gas in
J/g–K. The latent heat of vaporization of water = 540 cal/g.
[Ans. 0.90 J/g–K]
![]() (a) the final pressure to the initial pressure and (b) the final temperature to the initial temperature.
(a) the final pressure to the initial pressure and (b) the final temperature to the initial temperature.
[Ans. (a) 1.54, (b) 1.15]
17. An ideal gas at pressure 2.5 × 105 Pa and temperature 300K occupies 100 cc. It is adiabatically
compressed to half its original volume. Calculate
(a) the final pressure,
(b) the final temperature and
(c) the work done by
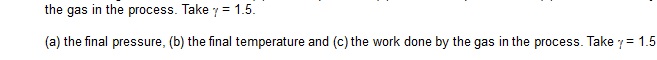
[Ans. (a) 7.1 × 105 Pa, (b) 424 K, (c) –21 J]
![]() the temperature of the air coming out of the tyre. Neglect any mixing with the atmospheric air.
the temperature of the air coming out of the tyre. Neglect any mixing with the atmospheric air.
[Ans. 240 K]
19. A gas is enclosed in a cylindrical can fitted with a piston. The walls of the can and the piston are adiabatic.
The initial pressure, volume and temperature of the gas are 100 kPa, 400 cm3 and 300 K respectively.
The ratio of the specific heat capacities of the gas is Cp/Cv = 1.5. Find the pressure and the temperature of the gas if it is
(a) suddenly compressed
(b) slowly compressed to 100 cm3.
[Ans. 800 kPa, 600K in both cases]
![]() with surrounding
with surrounding
. (a) It is slowly compressed to a volume V0/2 and then suddenly compressed to V0/4. Find the final pressure.
(b) If the gas is suddenly compressed from the volume V0 to V0/2 and then slowly compressed to V0/4, what will be the final pressure?
[Ans. 2g+1 p0 in both cases]
![]()
isothermally taken to a pressure p0/2 and from there adiabatically to a pressure p0/4. Find the final volume.
(b) The gas is brought back to its initial state. It is adiabatically taken to a pressure p0/2 and from there isothermally to a pressure p0/4. Find the final volume.
![]()
pressure and the initial temperature are 150 kPa and 300 K. Find
(a) the number of moles of the gas in the sample,
(b) the molar heat capacity at constant volume,
(c) the final pressure and temperature,
(d) the work done by the gas in the process and
(e) the change in internal energy if the gas.
[Ans. (a) 0.009, (b) 2R = 16.6 J/mol–K, (c) 780 kPa, 520 K, (d) –33 J, (e) 33 J]
![]()
each sample is doubled, the process being isothermal for A, adiabatic for B and isobaric for C.
If the final pressures are equal for the three samples, find the ratio of the initial pressures.
![]()
24. Two samples A and B of the same gas have equal volumes and pressures. The gas in sample A is expanded
isothermally to double its volume and the gas in B is expanded adiabatically to double its volume. If the work
![]()
of the final pressure to the initial pressure.
(b) If the original pressure is 100 kPa, find the work done by the gas in the process.
(c) What is the change in internal energy?
(d) What is the final temperature?
(e) The gas is now cooled to 300 K keeping its pressure constant. Calculate the work done during the process.
(f) The gas is now expanded isothermally to achieve its original volume of 1 litre. Calculate the work done by the gas.
(g) Calculate the total work done in the cycle.

27. Figure shows two rigid vessels A and B each of volume 200 cm3 containing an ideal gas (Cv=12.5 J/mol–K).
The vessels are connected to a monometer tube containing mercury. The pressure in both the vessels is 75cm
of mercury and the temperature is 300 K.
(a) Find the number of mole of the gas in each vessel.
(b) 5.0 J of heat is supplied to the gas in the vessel A and 10 J to the gas in the vessel B. Assuming no appreciable
transfer of heat from A to B calculate the difference in the heights of mercury in the two sides of the manometer.
Gas constant R = 8.3 J/mol–K.
[Ans. (a) 0.008, (b) 12.5 cm]
![]()
gases are equal. The gases are electrically heated for some time during which equal amounts of heat are given
to the two gases. It is found that the temperatures rise through the same amount in the two vessels.
Calculate the mass of hydrogen.
[Ans. 0.03 g]

and pressures in the two vessels. (b) The valve is now opened for sufficient time so that the gases
acquire a common temperature and pressure. Find the new values of the temperature and the pressure.

![]()
31. An adiabatic cylindrical tube of cross-sectiona area 1 cm2 is closed at one end and fitted with a piston at the other end.
The tube contains 0.03g of an ideal gas. A 1 atm pressure and at the temperature of the surrounding, the length of the gas
column is 40 cm. The piston is suddenly pulled out to double the length of the column. The pressure of the gas falls to
0.355 atm. Find the speed of sound in the gas at atmospheric temperature.
[Ans. 447 m/s]
32. The speed of sound in hydrogen at 0ºC is 1280 m/s. The density of hydrogen at STP is 0.089 kg/m3.
Calculate the molar heat capacities Cp and Cv of hydrogen.
[Ans. 18.0 J/mol–K, 26.3 J/mol–K]
33. 4.0 g of helium occupies 22400 cm3 at STP. The specific heat capacity of helium at constant pressure is 5.0 cal/mol–K.
Calculate the speed of sound in helium at STP.
[Ans. 960 m/s]
34. An ideal gas having density 1.7 × 10–3 g/cm3 at a pressure 1.5 × 105 Pa is filled in a Kundt’s tube. When the gas is resonated at frequency
of 3.0kHz, nodes are formed at a separation of 6.0 cm. Calculate the molar heat capacities Cp and Cv of the gas.
[Ans. 26 J/mol–K, 17.7 J/mol–K]
35. Standing waves of frquency 5.0 kHz are produced in a tube filled with oxygen at 300 K. The separation between the
consecutive nodes us 3.3 cm. Calculate the specific heat capacities Cp and Cv of the gas.
[Ans. 29.0 J/mol–K, 20.7 J/mol–K]