Solved Examples
43.1 Calculate the energy of a He+ ion in its first excited state.
![]()
For a He+ ion, Z = 2 and for the first excited state, n = 2 so that the energy of He ion in the first excited state is – 13.6 eV.
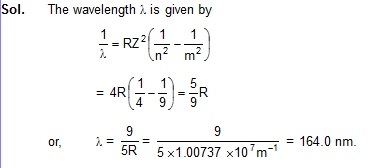
43.2 Calculate the wavelength of radiation emitted when He+ makes a transition from the state n = 3 to the state n = 2.
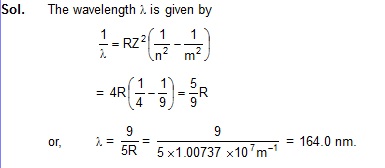
43.3 The excitation energy of hydrogen-like ion in its first excited state in 40.8 eV. Find the energy needed to
remove the electron from the ion.

Questions for Short answer
1. How many wavelengths are emitted by atomic hydrogen in visible range (380 nm – 780 nm)?
In the range 50 nm to 100 nm ?
2. The first excited energy of a He+ ion is the same as the ground state energy of hydrogen. Is it always
true that one of the energies of any hydrogen-like ion will be the same as the ground state energy of a hydrogen atom ?
3. Which wavelengths will be emitted by a sample of atomic hydrogen gas (in ground state) if electrons
of energy 12.2 eV collide with the atoms of the gas ?
4. When white radiation is passed through a sample of hydrogen gas at room temperature, absorption lines are
observed in Lyman series only. Explain.
5. Balmer series was observed and analysed before the other series. Can you suggest a reason for such an order?
6. What will be the energy corresponding to the first excited state of a hydrogen atom if the potential energy of
the atom is taken to be 10 eV when the electron is widely separated from the proton? Can we still write widely
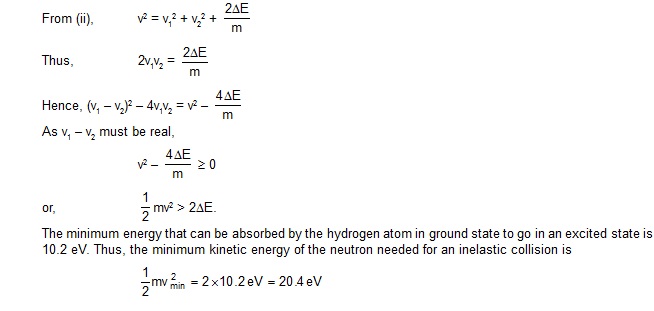
eparated from the proton? Can we still write En = E1/n2? rn = a0 n2?
7. The difference in the frequencies of series limit of Lyman series and Balmer series is equal to
the frequency of the first line of the Lyman series.. Explain.
8. The numerical value of ionization energy in eV equals the ionization potential in volts.
Does the equality hold if these quantities are measured in some other units?
9. We have stimulated emission and spotaneous emission. Do we also have stimulated absorption and spontaneous absorption?
10. An atom is in its excited state. Does the probability of its coming to ground state depend
on whether the radiation is already present or not? If yes, does it also depend on the wavelength of the radiation present?
Objective - I
1. The minimum orbital angular momentum of the electron in a hydrogen atom is -
(A) h (B) h/2 (C*) h/2pin (D) h/
2. Three photons coming from excite3d atomic-hydrogen sample are picked up
Their energies are 12.1 V, sample 10.2eV and 1.9eV. These photons must come from
(A) a single atom (B) two atoms (C) three atom (D*) either two atoms or three atoms
3. Suppose, the electron in a hydrogen atom makes transition from n = 3 to n = 2 in 10–8 s. The order of the torque acting
on the electron in this period, using the relation between troque and angular momentum as discussed in
the chapter on rotational mechanics is
(A) 10-34 N-m (B*) 10-24 N-m (C) 10-42 N-m (D) 10-8 N-m
4. In which of the following transitions will the wavelength be minimum ?
(A) n = 5 to n = 4 (B) n = 4 to n = 3 (C) n = 3 to n = 2 (D*) n = 2 to n = 1
5. In which of the following systems will the radius of the first orbit (n=1) be minimum ?
(A) hydrogen atom (B) deuterium atom
(C) singly ionized helium (D*) doubly ionized lithium
6. In which of the following systems will the wavelength corresponding to n=2 to n=1 be minimum ?
(A) hydrogen atom (B) deuterium atom
(C) singly ionized helium (D*) doubly ionized lithium
7. Which of the following curves may represent the speed of the electron in a hydrogen atom
as a function of the principal quantum number ?
 31.JPG)
8. As one considers orbits with higher values of n in a hudrogen atom, the electric potential energy of the atom
(A) decreases (B*) increases (C) remains the same (D) does not increase
9. The energy of an atom (or ion) in its ground state is - 54.4eV. It may be
(A) hydrogen (B) deuterium (C*) He (D) Li
10. The radius of the shortest orbit in a one-electron system is 18 pm. It may be
(A) hydrogen (B) deuterium (C) He (D*) Li
11. A hydrogen atom in ground state absorbs 10.2eV of energy. The orbital angular momentum of the electron is increased by
(A*) 1.05 x 10 -34 J-s (B) 2.11 x 10 -34 J-s (C) 3.16 x 10 -34 J-s (D) 4.22 x 10 -34 J-s
12. Which of the following parameters are the same for all hydrogen-like atoms and ions in their ground states ?
(A) radius of the orbitq (B) speed of the electron
(C) energy of the atom (D*) orbital angular momentum of the electron
13. In a laser tube, all the photons
(A) have same wavelength (B) have same energy
(C) move in same direction (D*) move with same speed
Objective - II
1. In a laboratory experiment on emission from atomic hydrogen in a discharge tube, only a small number
of lines are abserved whereas a large number of lines are present in the hydrogen spectrum of a star. This is because in a laboratory
(A) the amount of hydrogen taken in much smaller than that present in the star.
(B*) the temperature of hydrogen is much smaller than that of the star
(C) the pressure of hydrogen is much smaller than that of the star
(D) the gravitational pull is much smaller than that in the star.
2. An electron with kinetic energy 5 eV is incident on a hydrogen atom in its ground state. The collision
(A*) must be elastic (B) may be partially elastic
(C) must be completely inelastic (D) may be completely inelastic
3. Which of the following products in a hydrogen atom are independent of the principal quantum n ? The symbols have their usual meanings
(A*) un (B*) Er (C) En (D) ur
4. Let An be the area enclosed by the nth orbit in a hydrogen atom. The graph of ln (An / A1) against ln (n)
(A*) will pass through the origin (B*) will be a straight line with slope 4
(C) will be a monotonically increasing nonlinear curve (D) will be a circle
Sol. Q rn = n2r1
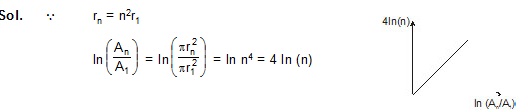
5. Ionization energy of a hydrogen-like ion B. Let r, u, E and L represent the radius of the orbit, speed
of the electron, energy of the atom and orbital angular momentum of the electron respectively. In ground state
(A) rA > rB (B*) uA > uB (C) EA > EB (D) LA > LB
6. When a photon stimulates the emission of another photon, the two photons have
(A*) same energy (B*) same direction (C*) same phase (D*) same wavelength
Worked Out Examples
1. Find the radius of Li++ ions in its ground state assuming Bohr’s model to be valid.
cksgj ds ekWMy dks lR; ekurs gq, Li++ vk;u dh f=kT;k dh x.kuk dhft,A HCV_Ch-43_WOE_1
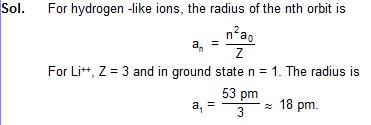
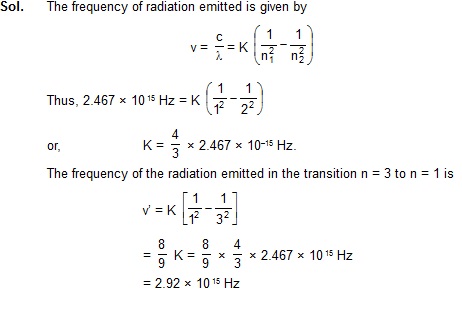
2. A particular hydrogen-like ion emits radiation of frequency 2.467 × 1015 Hz when it makes transition from
n = 2 to n = 1. What will be the frequency of the radiation emitted in a transition from n = 3 to n = 1?
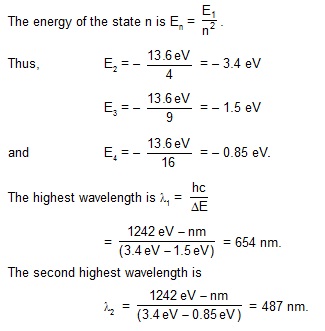
3. Calculate the two highest wavelengths of the radiation emitted when hydrogen atoms make transitions
from higher states to n = 2 states.
Sol. The highest wavelength corresponds to the lowest energy of transition. This will be the case for
the transition n = 3 to n = 2. The second highest wavelength corresponds to the transition n = 4 to n = 2.
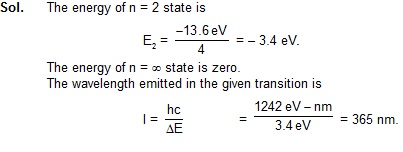
4. What is the wavelength of the radiation emitted when the electron in a hydrogen atoms jumps from n = infinite to n = 2?
. 
5. (a) Find the wavelength of the radiation required to excite the electron in Li++ from the first to the third Bohr orbit.
(b) How many spectral linea are observed in the emission spectrum of the above excited system?
Sol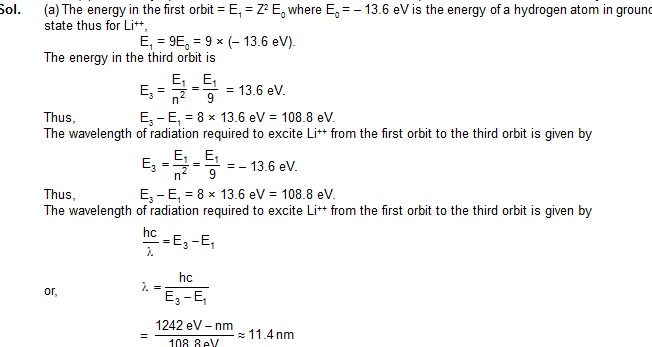
![]()
6. Find the wavelengths present in the radiation emitted when hydrogen atoms excited to n = 3 states return to their ground states.
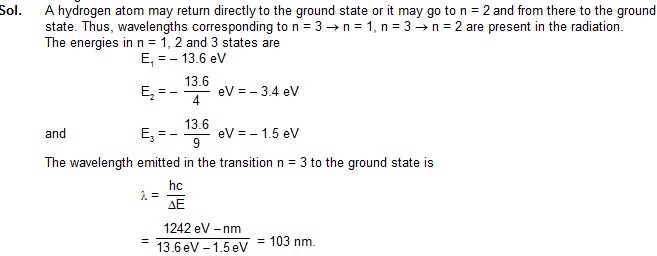
7. How may different wavelengths may be observed in the spectrum from a hydrogen sample if the
atoms are excited to states with principal quantum number n ?
Sol. From the nth state, the atom may go to (n – 1)th state, ...., 2nd state or 1st state. So there are
(n – 1) possible transitions starting from the nth state. The atoms reaching (n – 1)th state may make
(n – 2) different transitions. Similarly for other lower states. The total number of possible transitions is
(n – 1) + (n – 2) + (n – 3) +............2 + 1
= n (n-1) / 2
. 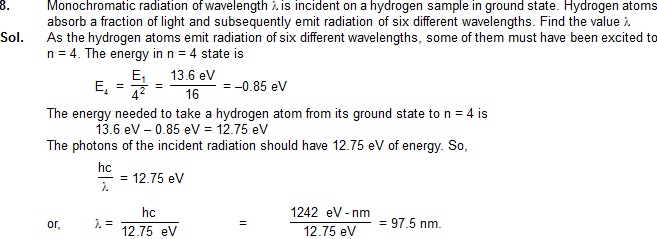
9. The energy needed to detach the electron of a hydrogen–like ion in ground state is 4 rydberg.
(a) What is the wavelength of the radiation emitted when the electron jumps from the
first excited state to the ground state ?
(b) What is the radius of the first orbit for this atom ?
Sol. (a) ln energy units,1 rydberg =13.6ev. The energy neededto detach the electron is 4×13.6ev.
The energy in the ground state is, therefore,E1=-4×13.ev. The energy of the first excited state(n=2)
is E2=E1/4=13.6ev=40.8eV The wavelengen of the radiationemitted is
![]()
(b) The energy of a hydrogen-like ion in ground state is E=Z2E0 where Z= atomic unmber and E0 = -13.6eV
Thus, Z=2. The eadius of the first orbitis a0/Z where a0 =53 pm. Thus,
r = 56 pm / 2 = 26.5pm
10. A hydrogen sample is prepared in a particular excited state A. Photons of energy 2.55 eV get absorbed into the
sample to take some of the electrons to a further excited state B. Find the quantum numbers of the states A and B.
Sol. The allowed energies of hydrogen atoms are
E1 = – 13.6 eV
E2 = –3.4 eV
E3 = –1.5 eV
E4 = –0.85 eV
E5 = – 0.54 eV
We see that a different of 2.55 eV can only be absorbed in transition n = 2 to n = 4. So the state A has quantum
number 2 and the state B has quantum number 4.

or l0 = 1242 eV- nm /13.6 eV= 91.3 nm,

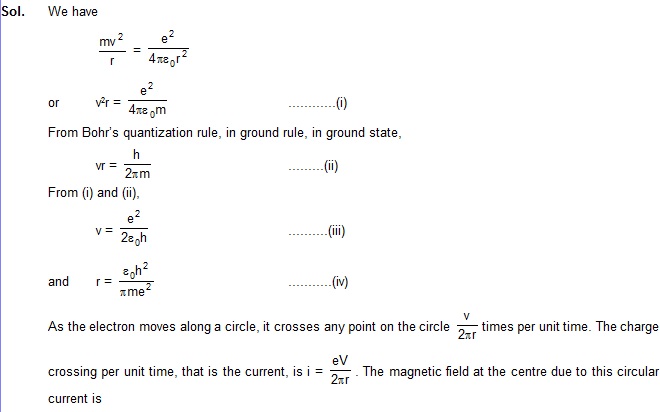
12. Derive an expression for the magnetic field at the site of the nucleus in a hydrogen atom due to the
circular motion of the electron. Assume that the atom is in its ground state and give the answer in terms
of fundamental constants.
. 
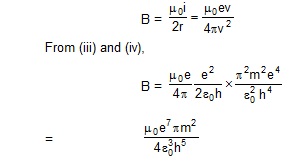
13. A lithium atom has three electrons, Assume the following simple picture of the atom. Two electrons move close to
the nucleus making up a spherical cloud around it and the third moves outside this cloud in a circular orbit. Bohr’s
model can be used for the motion of this third electron but n = 1 states ar not available to it. Calculate the ionization
energy of lithium in ground state using the above picture.
Sol. In this picture, the third electron moves in the field of a total charge + 3e – 2e = + e. Thus, the energies are
the same as that of hydrogen atoms. The lowest energy is :
![]()
Thus, the ionization energy of the atom in this picture is 3.4 eV.
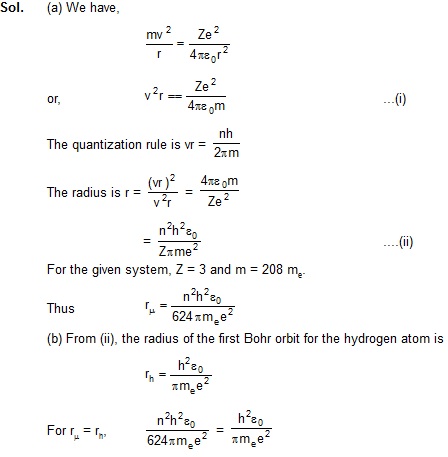
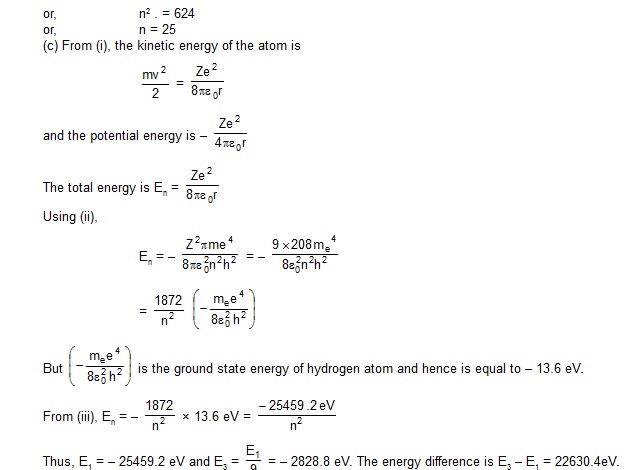
14. A particle known as -meason, has a charge equal to that of an electron and mass 208 times the mass of the electron.
It moves in a circular orbit around a nucleus of charge +3e.
Take the mass of the nucleus to be infinite. Assuming that the Bohr’s model is applicable to this system, (a) derive an
expression for the radius of the nth Bohr orbit, (b) find the value of n for which the radius of the orbit is approximately
the same as that of the first Bohr orbit for a hydrogen atom and (c) find the wavelength of the radiation emitted when the
–meson jumps from the third orbit to the first orbit.

The energy difference between the statesn involved in the transition should, therefore, br between 1.77eVand 2.44 eV.
_________________ n=4, E=-0.85eV _________________ n=3,E= -1.5eV _________________ n=2, E= -3.4eV _________________n=1,E= -13.6eV
Figure shoes same of the energies of hydrogen states. It is clear that only those transition which end at n= 2 may
emit photons of energy between 1.77 eV the proper range. The energy of the photon emitted in the transition n
= 3 to n=2 is DE=(3.4 - 1.5 ) eV= 1.9eV. The wavelenght is
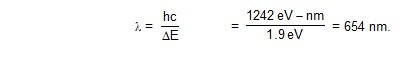
16. A beam of ultraviolet radiation having wavelength between 100 nm and 200 nm is incident on
a sample of atomic hydrogen gas. Assuming that the atoms are in ground state, which wavelength
will have low intensity in the transmitted beam? If the energy of a photon is equal to the difference
between the energies of an excited being absorbed by an atom in the ground state.
Sol. The energy of a photon corresponding to l = 100 nm is
1242eV- nm / 100 nm = 12.42 eV
and that corresponding to l = 200 nm is 6.21 eV.
The energy needed to take the atom from the ground state to the first excited state is
E2 – E1 = 13.6 eV – 3.4 eV = 10.2 eV,
to the second excited state is
E3 – E1 = 13.6 eV – 1.5 eV = 12.1 eV.
to the third excited state is
E4 – E1 = 13.6 eV – 0.85 eV = 12.75 eV etc.
Thus, 10.2 eV photons and 12.1 eV photons have large probability of being absorbed from
the given range 6.21 eV to 12.42 eV. The corresponding wavelengths are
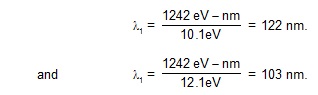
These wavelengths will have low intensity in the transmitted beam.
17. A neutron moving with speed v makes a head-on collision with a hydrogen atom in ground
state kept at rest. Find the minimum kinetic energy of the neutron for which inelastic (completely or partially)
collision may take place. The mass of neutron = mass of hydrogen = 1.67 × 10–27 kg.
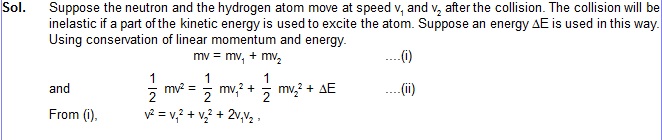
18. Light corresponding to the transition n = 4 to n = 2 in hydrogen atoms falls on cesium metal
(work function = 1.9 eV). Find the maximum kinetic energy of the photoelectrons emitted.
Sol. The energy of the photons emitted in transition n = 4 to n = 2 is
hv = 13.6 eV[1 /22 -1 /42] = 2. 55 eV
The maximum kinetic energy of the photoelectrons is
= 2.55 eV – 1.9 eV = 0.65 eV.
Exercise
2. Find the wavelength of the radiation emitted by hydrogen in the transitions (a) n = 3 to n = 2,
(b)n = 5 to n = 4 and (c) n = 10 to n = 9.
Ans: (a) 654 nm (b) 4050 nm (c) 38860
3. Calculate the smallest wavelength of radiation that may be emitted by (a) hydrogen, (b) He + and (c) Li++
Ans: (a) 91 nm (b) 23 nm (c) 10 nm
4. Evaluate Rydberg constant by putting the values of the fundamental constants in its expression.
Ans : 1.097 × 10 7 m – 1
5 Find the binding energy of a hydrogen atom in the state n = 2.
Ans : 3.4 eV
6. Find the radius and energy of a He+ ion in the states (a) n = 1, (b) n = 4 and (c) n = 10.
Ans : (a) 0.265 A, – 54.4 eV (b) 4.24 A ,– 3.4 eV
7. A hydrogen atom emits ultraviolet radiation of wavelength 102.5 nm. What are the quantum
numbers of the states involved in the transition ?
Ans : 1 and 3
8. (a) Find the first excitation potential of He+ ion (b) Find the ionization potential of Li++ ion.
Ans : (a) 40.8 V (b) 122.4 V
9. A group of hydrogen atoms are prepared in n = 4 states. List the wavelength that are emitted
as the atoms make transitions and return to n = 2 states .
Ans : 487 nm, 654 nm 1910 nm
10. A positive ion hydrogen just one electron ejects it if a photon of wavelength 228 Å
of less is absorbed by it identify the ion.
Ans : He +
11. Find the maximum coulomb force that can act on the electron due to the nucleus in a hydrogen atom.
Ans: 8.2 × 10 – 8 N
12. A hydrogen atom in a state having a binding energy of 0.85 eV makes transition to a state with excitation
energy 10.2 eV (a) identify the quantum numbers n of the upper and the lower energy states involved in the
ransition. (b) Find the wavelength of the emitted radiation.
Ans: (a) 4, 2 (b) 487 nm
13. Whenever a photon is emitted by hydrogen in Balmer series it is followed by another photon in Lyman series.
What wavelength does this latter photon correspond to ?
Ans: 122 nm
14. A hydrogen atom in stage n = 6 makes two successive transitions and reaches the ground state.
In the first transition a photon of 1.13 eV is emitted in the second transition (b) What is the value of n
in the intermediate state?
Ans: 121.eV , 3
15. What is the energy of a hydrogen atom in the first excited state if the potential energy
is taken to be zero in the ground state ?
Ans: 23.8 eV
15. What is the energy radiation of wavelengths 46.0 nm, 82.8 nm and 103.5 nm only. Assume that the atoms
have only two excited states and the difference between consecutive energy levels decreases as energy is increased.
Taking the energy of the highest energy state to be zero, find the energies of the ground state and the first excited state.
16. A hot gas emits radiation of wavelengths 46.0 nm 82.8 nm and 103.5 nm only. Assume that the atoms have only
two excited states and the difference between consecutive energy levels decreases as energy is increased.
Taking the energy of the ground state and the first excited state.
Ans: – 27 eV , – 12 eV
17. A gas of hydrogen like ions is prepared in a particular excited state A it emits photons having wavelength
equal to the wavelength of the first line of the lyman series together with photons of five other wavelength
identify the gas and find the principal quantum number of the state A.
Ans: He + 4,
18. Find the maximum angular speed of the electrons of a hydrogen atom in a stationary orbit.
Ans: 4.1 × 10 16 rad/s
Ans: 38
20. Suppose in certain conditions only those transitions are allowed to hydrogen atoms in which the principal
quantum number n change by 2 (a) Find the smallest wavelength emitted by hydrogen (b) List the
wavelengths emitted by hydrogen in the visible range (380 nm to 780)
Ans: (a) 103 nm (b) 487 nm
21. According to Maxwell’s theory of electrodynamics an electrons going in a circle should emit radiation
of frequency equal to its frequency of revolution .What should be the wavelengths of the radiation emdiation
by a hydrogen atom in ground state if this rule is followed ?
Ans: 45.7nm
22. The average lometic energy of molecules in a gas at temperature T is KT .Find the temperature at which the
average kinetic energy of the molecules of hydrogen equals the binding energy of its atoms will hydrogen
remain in molecular form at this temperature ?Take K = 8.62 × 10– 5 eV/K
Ans: 1.05 × 10 5 K
23. Find the temperature at which the average thermal kinetic energy is equal to the energy needed to take a
hydrogen atom from its ground state to n = 3 state. Hydrogen can now emit red light of wavelengths 653.1nm
Because of Maxwellian distributions of speeds, a hydrogen sample emits red light at temperature much lower
than that obtained from this problem. Assume that hydrogen molecules dissociate into atoms.
Ans: 9.4 × 10 4 K
24. Average lifetime of a hydrogen atom excited n = 2 state is 10– 8 s. Find the number of revolutions
made by the electrons on the average before it jumps to ground state.
Ans: 8.2 × 10 6
25. Calculate the magnetic dipole moment corresponding to the motion of the electrons in the ground state of a hydrogen atom.
Ans: 9.2 × 10
26. Show that the ratio of the magnetic dipole moment to the angular momentum (l = mnr) is universal
constant for hydrogen like atoms and ions. Find its value.
Ans: e/2m8.8×1010c/kg
27. A beam of light having wavelengths distributed uniformly between 450 nm to 550 passes through
a sample of hydrogen gas. Which wavelength will have the least intensity in the transmitted beam ?
Ans: 487 nm
28. Radiation coming from transitions n = 2 to n = 1 of hydrogen atoms falls on helium ions in n = 1
and n =2 states. What are the possible transitions of helium ions as they absorb energy from the radiation?
Ans: n = 2 to n = 3 and n = 2 to n = 4
29. A hydrogen atom in ground state absorbs a photon of ultraviolet radiation of wavelength 50 nm Assumin
that the entire photon energy is taken up by the electron with what kinetic energy will the electrons be ejected ?
Ans : 11.24 eV
30. A parallel beam of light of wavelength 100nm passes through a sample of atomic hydrogen gas in ground stat
(a) Assume that when a photon supplies some of its energy to a hydrogen atom the rest of the energy by the excited
hydrogen atoms in the direction of the incident beam what wavelengths may be observed in the transmitted beam ?
(b) A radiation detector is placed neat the gas to detect radiation coming perpendicular to the incident beam.
Find the wavelengths of radiation that may be detected by the detector.
Ans: (a) 100 nm , 560 nm 3880 (b) 103 nm, 121 nm 654 nm
31. A beam of monochromatic light of wavelength l ejects photoelectrons from a cesium surface (f = 1.9 eV)
These photoelectrons are made to collide with hydrogen atoms in ground state .Find the maximum value of l
for which (a) hydrogen atoms may be ionised (b) hydrogen atoms may get excited from the ground state to the
first excited state and (c) the excited hydrogen atoms may emit visible light.
Ans: (a) 80 nm (b) 102 nm (c) 89 nm
32. Electrons are emitted from an electron gun at almost zero velocity and accelerated by an electric field
E through a distance of 1.0 m The electrons are now scattered by an atomic hydrogen sample in ground state.
What should be the minimum value of E so that red light of wavelength 656 .3 may be emitted by the hydrogen ?
Ans: 12.1 V/m
33. A neutron having kinetic energy 12.5 eV collides with a hydrogen atom at rest Neglect the differenc
in mass between the neutron and the hydrogen atom and assume that the neutron does not leaves its
line of motion Find the possible kinetic energies of the neutron after the event.
Ans: zero
34. A hydrogen atom moving at speed n colloids with another hydrogen atom kept at rest Find the
minimum value of n for which one of the atoms may get ionized the mass of a hydrogen atom = 1.67 × 10–27 kg.
Ans: 7.2 × 10 4 m/s
35. A neutron moving with a speed v strikes a hydrogen atom in ground state moving towards it with the same speed.
Find the minimum speed of the neutron for which inelastic (completely of partially) collision may get ionized
The mass of neutron = mass of hydrogen = 1.67 × 10– 27kg
Ans: 3.13 × 104 m/s
36. When a photons is emitted by a hydrogen atom, the photon carries a momentum with it
(a) Calculate the momentum carried by the photon when a hydrogen atom emits light of wavelength 656.3nm
(b) With what speed does the atom recoil during this transition ?Take the mass of the hydrogen
atom =1.67 × 10 – 27 kg (c) Find the kinetic energy of recoil of the atom
Ans : (a) 1.0 × 10 27 kg m/s (b) 0.6 m/s
37. When a photon is emitted from an atom the atom recoils. The kinetic energy of recoil and the energy of the
photon come from the difference in energies between the states involved in the energies between the states
involved transition suppose a hydrogen atom changes its state from n = 3 to n = 2 Calculate the fractional
change in the wavelength of light emitted, due to the recoil
Ans: 10 – 9
38. The light emitted in the transition n = 3 to n = 2 in hydrogen is called Ha light. Find the maximum
work function a metal can have so that Ha light can emit photoelectrons from it.
Ans: 1.9 eV
39. Light from Balmer series of hydrogen is able to eject photoelectrons from a metal .
What can be the maximum work function of the metal?
Ans: 3.4 eV
40. Radiation from hydrogen discharge tube falls on a cesium plate. Find the maximum possible
kinetic energy of the photoelectrons .Work function of cesium is 1.9 eV.
Ans: 11.7 eV
41. A filter transmits only the radiation of wavelength greater than 440 nm. Radiation from a hydrogen
discharge tube goes through such a filter and is incident on a metal of work function 2.0 eV Find the stopping
potential which can stop the photoelectrons.
Ans: 0.55
42. The earth revolves round the sun due to gravitational attraction Suppose that the sun and the earth are point
particles with their existing masses and that Bohar ‘s quantization rule for angular momentum is valid in the
case of gravitation (a) Calculate the minimum radius the earth can have for its orbit (b) What is the value of the
principal quantum number n for the present radius ? Mass of the earth = 6.0 ×1024 kg mass of the sun 2.0 × 1030
kg earth sun distance = 1.5 × 1011m.
Ans: (a) 2.3 × 10 – 138 m (b) 2.5 × 10 74
43. Consider a neutron and an electrons bound to each other due to gravitational force .Assuming Bohar s
quantization rule for angular momentum to be valied in this case derive an expression for the energy of
the neutron - electron system.
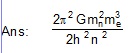
44. A uniform magnetic field B exists in a region An electron projected perpendicular to the field goes in a
circle Assuming Bohar’s quantization rule for angular momentum calculate
(a) the smallest possible radius of the electron (b) the radius of the nth orbit and
(c) the minimum possible speed of the electron.
![]()
45. Suppose in an imaginary world the angular momentum is quantized to be even integral multiples of h/2p
What is the longest possible wavelength emitted by hydrogen atoms in visible range in such a world according to Bohr’s model?
Ans: 487 nm
46. Consider an excited hydrogen atom in state n moving with a velocity n (n << c ).It emits a photons in
the direction of its motion and changes its state to a lower state m Apply momentum and energy
conservation principles to calculate the frequency n of the emitted radiation compare this wite the
frequency vo emitted if the atom were at rest.
![]()